FIG. 12.
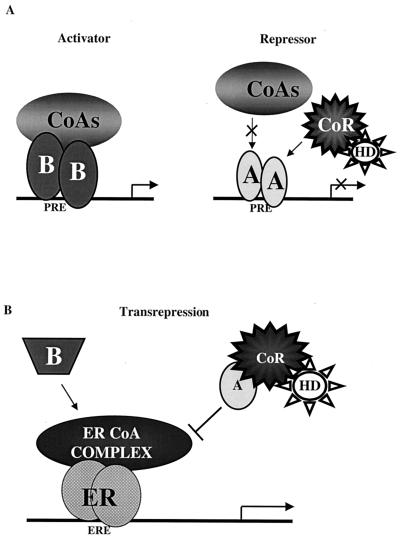
Two distinct models are required to describe the molecular mechanism of action of hPRA. (A) Transcriptional activation. Based on the in vivo and the in vitro binding studies, we propose that hPRA interacts more efficiently with corepressors and less efficiently with coactivators than hPRB. In the presence of hormone, hPRB, but not hPRA, undergoes a favorable conformational change which allows it to displace corepressors (CoR) and recruit coactivator proteins (CoA), thus allowing hPRB to activate transcription from progesterone-responsive promoters. HD, histone deacetylase; A, hPRA; B, hPRB. (B) Transrepression. Based on our in vivo transrepression data, we propose that hPRA transrepresses ERα-mediated transcription by a transcriptional interference mechanism. In this model, ERα activates transcription by recruiting a complex of coactivator proteins (ERα CoA complex) to the regulatory region of target genes. hPRA (A), but not hPRB (B), targets and sequesters a member of the ERα CoA complex, thus preventing ERα from activating transcription. hPRA transrepression of ERα transcriptional activity is further enhanced by the recruitment by hPRA of the corepressor SMRT (CoR).