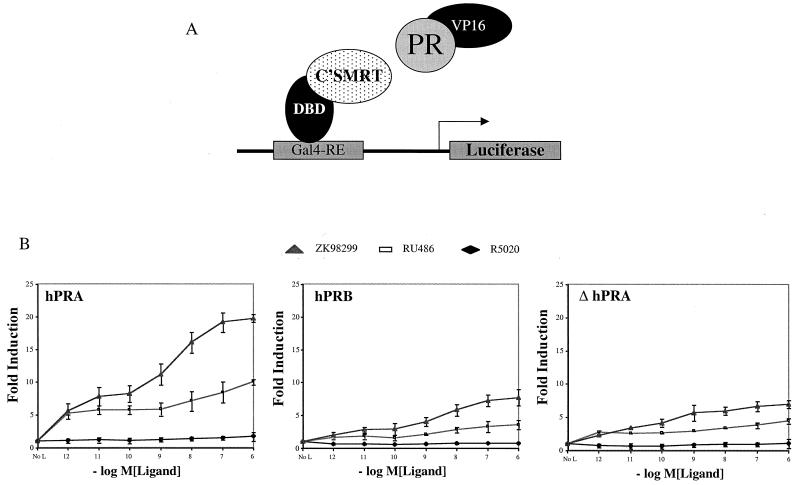
FIG. 4.
The ID facilitates hPRA's interaction with the corepressor SMRT. (A) Schematic of the mammalian two-hybrid assay. The receptor-interacting domain of SMRT (C'SMRT; aa 981 to 1495) was fused to the Gal4DBD (aa 1 to 147). hPRA, hPRB, or ΔhPRA was fused onto VP16 (VP16 acidic activation domain; aa 411 to 455). The fusion constructs were cotransfected into HeLa cells along with a reporter plasmid containing five copies of a Gal4-responsive element (Gal4-RE) upstream of the luciferase gene. (B) HeLa cells were transiently transfected with 0.5 μg of 5XGal4-TATA-LUC, 50 ng of pBKC-βgal, 1 μg of pCMX-Gal4-C'SMRT (Gal4-C'SMRT), 1 μg of either pVP16-hPRB, pVP16-hPRA, or pVP16-ΔhPRA, and 0.45 μg of pBSII-KS. Transcriptional activity was assayed on the 5XGal4-TATA-LUC reporter and represents an indirect measure of the binding of the fusion proteins. Transcriptional activity was measured following the addition of increasing concentrations of R5020, RU486, or ZK98299. Transfections were normalized for efficiency using an internal β-galactosidase control plasmid (pBKC-βgal). The data are represented as fold induction over the no-hormone (No L) response, which was set to 1.0. Each data point represents the average of triplicate determinations of the transcriptional activity under the given experimental conditions (n = 2).