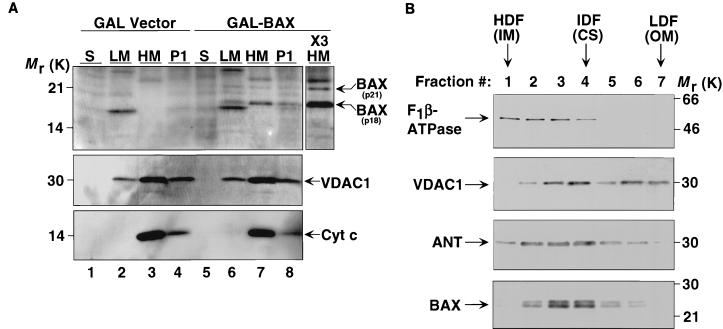
FIG. 2.
BAX localizes to mitochondria but does not cause detectable release of cyt c. (A) Subcellular distribution of BAX in yeast cells. Cells transformed with GAL vector (lanes 1 to 4) or with GAL-BAX (lanes 5 to 8) were suspended in isotonic buffer, homogenized, and separated into soluble fraction (S), light-membrane fraction (LM), heavy-membrane fraction (HM), and low-speed pellet (P1) by differential centrifugation. The fractions were analyzed by Western blotting using anti-mBAX Ab 651 (top panel), anti-yVDAC1 Ab (middle panel), and anti-cyt c MAb (PharMingen) (bottom panel). The P1 pellet contains residual whole cells, nuclei, and mitochondria. The HM fraction is enriched for intact mitochondria. The LM fraction contains the endoplasmic reticulum, plasma membrane, and mitochondrial fragments. The soluble (S) fraction represents the cytosol. ×3 HM denotes that three times the amount of protein loaded in lane 7 was loaded in this lane. (B) BAX is enriched in mitochondrial contact sites. Submitochondrial membrane vesicles were prepared from isolated yeast mitochondria expressing HA-BAX by sonication and separated by centrifugation through a continuous sucrose density gradient. Fractions were analyzed by Western blotting using anti-yF1β-ATPase Ab, anti-yVDAC1 Ab, anti-yANT Ab, and anti-mBAX Ab 651. The high-density fractions (HDF) are enriched in inner membrane (IM) vesicles. The low-density fractions (LDF) are enriched in outer mitochondrial membranes (OM). Intermediate-density fractions (IDF) are enriched for contact sites (CS), sites of association between the inner and outer membranes.