Abstract
Mitochondrial disorders are a heterogeneous group of rare, degenerative multisystem disorders affecting the cell’s core bioenergetic and signalling functions. Spontaneous improvement is rare. We describe a novel neonatal-onset mitochondriopathy in three infants with failure to thrive, hyperlactatemia, hyperammonemia, and apparent clinical resolution before 18 months. Exome sequencing showed all three probands to be identically heterozygous for a recurrent de novo substitution, c.620G>A [p.(Arg207His)] in ATP5F1A, encoding the α-subunit of complex V. Patient-derived fibroblasts exhibited multiple deficits in complex V function and expression in vitro. Structural modelling predicts the observed substitution to create an abnormal region of negative charge on ATP5F1A’s β-subunit-interacting surface, adjacent to the nearby β subunit’s active site. This disorder, which presents with life-threatening neonatal manifestations, appears to follow a remitting course; the long-term prognosis remains unknown.
Subject terms: Metabolic disorders, Medical genetics
Introduction
Mitochondrial disorders (MDs) are a group of >200 rare genetic diseases affecting cellular bioenergetics, metabolism and other essential processes [1]. A provisional genetic diagnosis can be made in ~25–50% of cases [2]. Although most MDs are progressive, spontaneous improvement is typical of two disorders, TRMU deficiency (OMIM #613070) and MTTE-related infantile mitochondrial myopathy (OMIM #500009) [3, 4]. Here, we describe a novel remitting MD in three neonates with failure to thrive, hyperammonemia, lactic acidosis, respiratory defects in fibroblasts, and recurrent de novo substitutions of the complex V α subunit gene, ATP5F1A.
Materials and methods
Procedures were compliant with the revised Helsinki Declaration of 2000. The study was approved by the Children’s Hospital of Eastern Ontario Research Ethics Board (#11/04E). All biochemical and genetic testing was performed routinely as part of usual care. Fibroblast cultures were obtained from all participants via routine skin biopsy. Non-MD control fibroblasts were obtained from a healthy volunteer (‘Control 1’), and from individuals with the non-mitochondrial conditions Fryns Syndrome (‘Control 2’) and 46,XY disorder of sexual development (‘Control 3’). Cell lines were assayed for complex V activity as described (Supplementary Material). Genetic variant information is accessible in ClinVar with variation ID 432972/accession VCV000432972.3.
Results
Clinical findings
The patients are three unrelated term neonates presenting between postnatal days nine and eighteen with feeding intolerance, failure to thrive, hyperammonemia and lactic acidemia (Table 1; Supplementary Fig. 1). Blood lactate was variably elevated at presentation (3.3–8.5 mmol/L). Metabolic tests showed hyperalaninemia (1245–1624 μmol/L), hyperprolinemia, hyperglutaminemia (two probands; 1725–1742 μmol/L), depletion of urea cycle intermediates (citrulline, arginine and ornithine) and orotic aciduria. Urine organic acids showed hyperexcretion of lactate with or without pyruvate and Krebs cycle intermediates. In one individual, hyperammonemia was mild (90 μmol/L) and resolved spontaneously; the other two children required protein restriction, intravenous fluids, dextrose, nitrogen-scavenging medications, and supplementary citrulline and/or arginine. Neither received haemodialysis, although both required hypercaloric, protein-restricted feeds and nitrogen-scavenging agents to maintain control of hyperammonemia. One individual was tested genetically for suspected ornithine transcarbamylase (OTC) deficiency, with normal results.
Table 1.
Clinical and laboratory features.
| Individual 1 | Individual 2 | Individual 3 | |
|---|---|---|---|
| Clinical presentation | |||
| Sex | M | F | F |
| Perinatal history | NRFHR, mild temperature instability | normal | NRFHR, C/S |
| Birth weight (SD) | −2.9 | +0.5 | −1.0 |
| Age at presentation | 18 days | 9 days | 13 days |
| Feeding difficulties | + | + | + |
| Failure to thrive | + | + | + |
| Acute encephalopathy | − | - | + (hyperammonemia) |
| Chronic diarrhoea | + | - | + |
| Anaemia | + | - | + |
| Age at most recent assessment | 3 years | 14 months | 18 months |
| Age at symptom resolution | 12 months | 10 months | 15–18 months |
| Clinical status | asymptomatic | asymptomatic | asymptomatic |
| Most recent weight (SD) | −1.0 | −0.8 | +0.2 |
| Developmental status | normal | normal | normal |
| Metabolic laboratory testinga | |||
| Hyperammonemia | + (267 μmol/L) | + (90 μmol/L) | + (520 μmol/L) |
| Encephalopathy | - | - | + (seizures after EBM gavage feeds) |
| Lactic acidosis (mmol/L) | + (range: 1.0–11.0; x̅ = 3.6 mmol/L) (n = 36) | + (range: 1.2–8.5 mmol/L; x̅ = 3.5 mmol/L) (n = 17) | + (range: 3.2–5.8 mmol/L; x̅ = 4.3 mmol/L) (n = 7) |
| Plasma amino acids (μmol/L): | |||
| Alanine | 1624 (↑↑) [83–447] | 1245 (↑↑) [119–439] | 1675 (↑↑) [75–500] |
| Proline | 530 (↑) [87–375] | 439 (↑) [104–348] | 590 (↑) [20–330] |
| Glycine | 574 (↑) [133–409] | 229 [103–386] | 530 [100–550] |
| Glutamine | 1742 (↑↑) [240–1194] | 979 [303–1459] | 1725 (↑) [124–1000] |
| Citrulline | BDL (↓) | BDL ( ↓) | 2 [0–50] |
| Arginine | 19 [6–140] | 18 (↓) [30–147] | 17 (↓) [50–160] |
| Ornithine | 23 (↓) [29–168] | NR | 18 (↓) [25–250] |
| Urine orotic acid (mmol/molCr) | 7.1 (↑) [<2.0] | NR | 9.3 (↑) [1.0–3.2] |
| Urine organic acids | Increased lactic, pyruvic, 2-ethyl-3-hydroxypropionic, ethylmalonic, Krebs cycle intermediates including fumaric | Increased lactic and pyruvic acids | Increased lactic acid |
| Acylcarnitine profile | Persistent (n = 4) minor increases in C3, C3DC, C4, C6, C6OH, and C10 | Normal (n = 3) apart from trivial increases in C6OH, C3:1, and/or C3 | Normal (n = 1) |
| Plasma GDF-15 (pg/mL) | >6000 (↑↑) [<750] (age 3 weeks) | 2030 (↑↑) [<750] (7 months) | NR |
| 3952 (↑↑) (age 2 months) | |||
| 3017 (↑↑) (age 5 months) | |||
| 2731 (↑↑) (age 13 months) | |||
| Residual/subclinical laboratory findings (at most recent assessment) | lactate 2.5 mmol/L; elevated GDF-15 (2731 pg/mL) (last tested age 13 months) | lactate 2.7 mmol/L; hyperalaninemia (685 μmol/L) | lactate 3.7 mmol/L |
| Muscle biopsy | |||
| Histology and EM | Increased lipid droplets, irregular loss of sarcomeres with replacement by glycogen, large, ‘swollen’ mitochondria with abnormal figures | NR | NR |
| Complexes I–IV | normal | ||
| Primary skin fibroblasts | |||
| Complex I | NR | NR | normal |
| Complexes II–IV | normal | NR | normal |
| PDH, PC and L/P ratio | normal | NR | NR |
Reference intervals are denoted in square brackets.
BDL below detectable limit, C/S Caesarean section, EBM expressed breast milk, F female, GDF-15 growth and differentiation factor 15, L/P lactate/pyruvate, M male, NR not reported, NRFHR non-reassuring foetal heart rate, PC pyruvate carboxylase, PDH pyruvate dehydrogenase, SD standard deviations.
aSee Supplementary Fig. 1 for additional details.
The patients experienced failure to thrive which proved refractory to anti-reflux medications, hypercaloric hypoallergenic feeds, gavage feeding, and (in one case) parenteral nutrition. Two individuals developed chronic non-bloody diarrhoea of indeterminate aetiology. Two of three probands had an unexplained anaemia. Pearson syndrome was considered in one case and duly excluded by mtDNA studies in muscle (see below).
Each proband was investigated for suspected mitochondriopathy. Plasma growth and differentiation factor 15 (GDF-15) was markedly increased in two individuals. Muscle biopsy in one case showed several histologic and ultrastructural abnormalities (Supplementary Fig. 2). Complex I–IV activities in muscle and lactate:pyruvate ratio in fibroblasts were normal. Mitochondrial DNA sequencing in muscle was negative for pathogenic point variants and rearrangements.
The major clinical symptoms appeared to remit by late infancy in all cases. Weight re-entered the normal range between ten and eighteen months; diarrhoea remitted before one year, and lactate stabilised near the upper normal limit after several months. One proband experienced recurrent hyperammonemia at age fifteen months, after single missed doses of her glycerol phenylbutyrate, arginine, and citrulline. As of their most recent assessment (age range: fourteen months to three years), the probands’ growth and developmental parameters are normal. They continue to exhibit subtle laboratory abnormalities including mild intermittent hyperlactatemia (three individuals), hyperalaninemia (one individual), and elevated GDF-15 (one individual). One proband (individual 3; age 18 months) remains on medical hyperammonemia prophylaxis with arginine, citrulline, and glycerol phenylbutyrate.
Exome sequencing
Clinical exome trio analysis (proband and parents) was performed in each case. All probands were identically heterozygous for the same de novo substitution, ATP5F1A(NM_001001937.1):c.620G>A, p.(Arg207His). This variant, which is absent from gnomAD [5], intersects all RefSeq transcripts of ATP5F1A, and carries pathogenic predictions (PHRED-scaled CADD 32; SIFT 0; PolyPhen-2 1.000).
Bioenergetic studies in patient fibroblasts
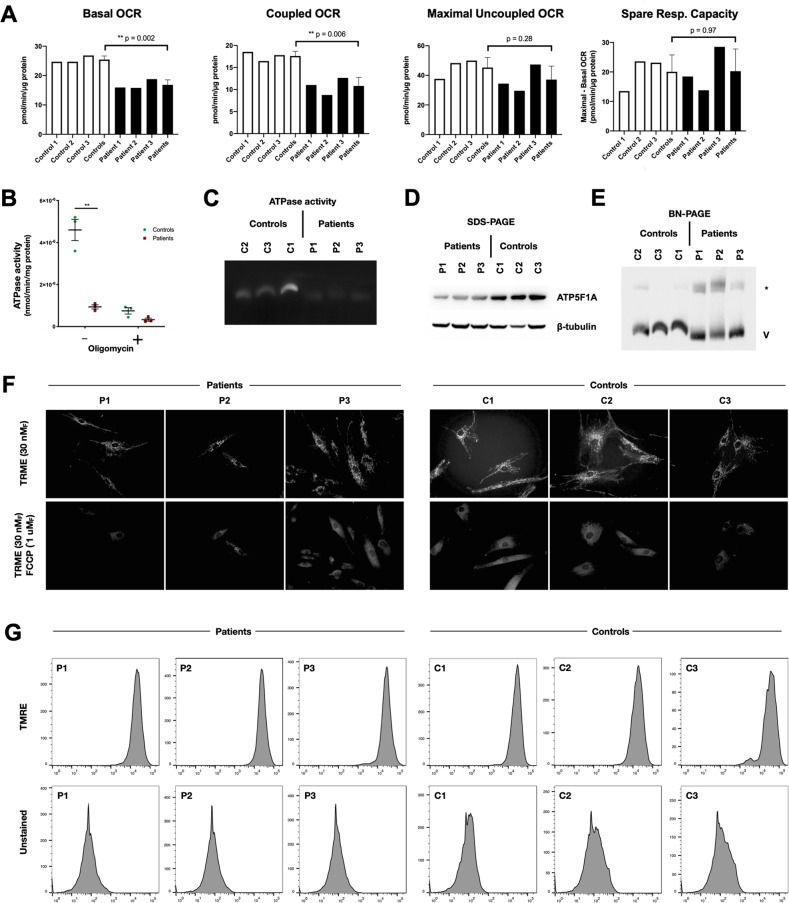
ATP5F1A encodes subunit α of mitochondrial ATP synthase (complex V), the rotary ATPase responsible for >90% of cellular ATP synthesis. To assess the functional consequences of the observed p.Arg207His substitution, we studied complex V activity in patient-derived fibroblasts using multiple methods (Fig. 1). Patient cells exhibited defects in mitochondrial oxygen consumption, ATPase activity, and ATP5F1A expression. Nondenaturing PAGE (Fig. 1E) further showed a smaller proportion of monomeric complex V in patient cells, with an increased proportion participating in higher-order complexes. The significance of the latter finding is unclear.
Fig. 1. Complex V defect in patient-derived fibroblasts.
a Extracellular flux analysis showed deficits in basal and coupled (complex V-mediated) oxygen consumption rate (OCR), but no difference in maximal uncoupled OCR or spare respiratory capacity. b Patient-derived fibroblasts exhibited reduced complex V activity measured as oligomycin-sensitive ATP synthase. c In-gel ATPase activity following nondenaturing electrophoresis was lower in patient-derived cells. d ATP5F1A expression was lower as assessed by immunoblot analysis. e Blue native PAGE showed a diminished proportion of monomeric complex V, and an increased abundance of a high-molecular-weight complex (*) of unknown composition. f Fluorescence microscopy of mitochondria stained with a voltage-sensitive cationic lipophilic dye (TMRE) with and without a pharmacological uncoupler (FCCP) demonstrated no difference in resting mitochondrial inner membrane potential in patient cells versus controls. Similarly, patient and control cells exhibited no significant difference in TMRE fluorescence intensity as assessed by flow cytometry (g).
Structural modelling
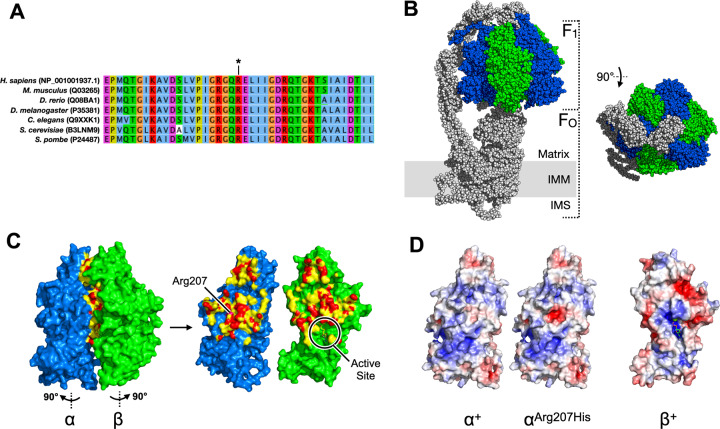
We modelled the potential structural consequences of the recurrent p.Arg207His substitution based on a previously-solved structure of ATP5F1A (Fig. 2) [6]. In the model, the sidechain of residue 207 resides at the α-β subunit interface, opposite the nearby β subunit’s active site. At matrix pH (~7.8), replacement of Arg207 (pKa 12.5) with a histidine (pKa 6.0) is a nonconservative substitution predicted to create a negatively-charged patch at the α–β interaction surface.
Fig. 2. Predicted functional consequence of p.Arg207His substitution in ATP5F1A.
The F1 catalytic core of complex V is a radial hexamer of alternating α and β subunits (α3β3) surrounding a central rotor (γδε). The three ATP-producing catalytic active sites lie 120° apart on each of the three β subunits, in clefts adjoining the β-α subunit interfaces. a Peptide sequences surrounding Arg207 (*) are highly conserved. b Cryo-EM structure of complex V (PDB: 6J5J) (ref. [18].). ATP5F1A (α) is shown in blue, and ATP51B (β) in green. c Detail of α-β subunit interface, per PDB:1COW (ref. [6].). Residues shown in red and yellow are within 3.6 Å and 5 Å, (respectively) of the opposing subunit. The β-subunit ATPase active site lies indirectly opposite α-Arg207 at the position shown. d at mitochondrial matrix pH (7.8), the β-interacting surface of αArg207His is predicted to display a region of central negative charge (red patch) not present in the unsubstituted protein.
Discussion
Complex V is uniquely crucial to cellular energy metabolism, and complex V disorders are very rare. This report of a comparatively mild condition extends the clinical spectrum of ATP5F1A as defined by two previous reports of lethal multisystem MD in children with biallelic disease [2, 7]. The other described genes for isolated complex V deficiency are ATP5F1D and ATP5F1E (encoding subunits δ and ɛ), TMEM70 and ATPAF2 (encoding assembly factors), and MT-ATP6 and MT-ATP8 (mitochondrial genes for subunits a and A6L of FO) [8–12]. Of the five nuclear complex V disorders, all except TMEM70 deficiency (~50 individuals) are rare congenital disorders comprising growth restriction, encephalopathy, cardiomyopathy, pulmonary hypertension, and/or congenital malformations. mtDNA-based forms of complex V deficiency are broadly variable by degree of heteroplasmy; nevertheless, these too are lifelong conditions with a significant associated burden of illness [12, 13].
The long-term prognosis of heterozygous ATP5F1A deficiency is unknown. All patients in this study were young, and subclinical biochemical abnormalities persisted in each case despite clinical resolution. Untreated, the condition described here is likely to be highly deleterious, considering: (i) its uniform presentation with life-threatening neonatal symptoms, (ii) absence of the causal variant in general-population databases, and (iii) de novo occurrence in each case. Given the nonspecific clinical presentation, exome or panel testing is anticipated to be the means of diagnosis in most cases. Clinical pitfalls relating to this disorder could include: (i) early assignment of an overly poor prognosis, (ii) overestimation of recurrence risks, (iii) misdiagnosis as ‘mutation-negative’ OTC deficiency, and (iv) false-negative respiratory chain testing where complex V is not specifically assessed.
Heterozygous ATP5F1A-related MD joins two other reversible neonatal MDs associated with the genes TRMU (OMIM #613070), and MTTE (OMIM #500009) [3, 4]. In MTTE-related mitochondriopathy, spontaneous recovery is proposed to entail activation of the integrated stress response via mTOR, an adaptive process [14]. In this study we did not observe the specific amino acid deficiencies (of cysteine, glutamine, and glutamate) described in MTTE deficiency [14].
The p.(Arg207His) substitution in our probands occurred de novo in each case. Possible explanations could include: (i) coincidence, (ii) selection bias towards similar cases, (iii) hypermutability of the specific genomic position (i.e., deamination of a CpG dinucleotide), or (iv) unique functional characteristics specific to the variant itself. At present, there is insufficient information to conclude whether the p.(Arg207His) substitution is hypomorphic versus dominant-negative. Although ATP5F1A is statistically depleted of truncating variants (gnomAD pLI: 1.00 and 0.98, respectively), multiple (>10) truncating variants are nevertheless found at low frequency among healthy adult controls [5]. In mice, homozygous Atp5f1a deficiency is embryonic-lethal; heterozygotes, although viable, are smaller versus controls [15, 16]. The possibility that ATP5F1Ap.(Arg207His) is dominant-negative with respect to complex V assembly, stability, or function remains to be tested in a suitable animal model.
Another MD gene with distinct recessive and dominant phenotypes is SLC25A4, encoding the ATP/ADP translocase (ANT1). In a manner similar to ATP5F1A, recurrent de novo substitutions of SLC25A4 have been shown to cause a congenital mitochondriopathy distinct from the adult-onset (monoallelic) and childhood-onset (biallelic) forms of ANT1 deficiency [17]. Because many mitochondrial inner membrane proteins function in macromolecular complexes, it stands to reason that structurally dominant-negative alleles may also occur in other, unrecognised, forms of MD.
Supplementary information
Acknowledgements
The authors gratefully acknowledge the participation of the patients and their families. Partial funding was received from Newborn Screening Ontario.
Author contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by M. L., A. C., T. N., M. L. D. L., J. M., C. P., T. T., N. S., H. C., F. M., and M. G. The first draft of the manuscript was written by M. L. and A. C., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability statement
All data generated or analysed during this study are included in this published article and its supplementary information files. Genetic variant information is accessible in ClinVar with variation ID 432972.
Ethical approval
Informed consent was obtained from all subjects, and study procedures were compliant with the revised Helsinki Declaration of 2000. The study was approved by the Children’s Hospital of Eastern Ontario Research Ethics Board (#11/04E).
Competing interests
Francisca Millan is employed by GeneDx (MD, USA); the remaining authors declare no conflicts.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-021-00956-0.
References
- 1.Vafai SB, Mootha VK. Mitochondrial disorders as windows into an ancient organelle. Nature. 2012;491:374–83. doi: 10.1038/nature11707. [DOI] [PubMed] [Google Scholar]
- 2.Lieber DS, Calvo SE, Shanahan K, Slate NG, Liu S, Hershman SG, et al. Targeted exome sequencing of suspected mitochondrial disorders. Neurology. 2013;80:1762–70. doi: 10.1212/WNL.0b013e3182918c40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeharia A, Shaag A, Pappo O, Mager-Heckel AM, Saada A, Beinat M, et al. Acute infantile liver failure due to mutations in the TRMU gene. Am J Hum Genet. 2009;85:401–7. doi: 10.1016/j.ajhg.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horvath R, Kemp JP, Tuppen HA, Hudson G, Oldfors A, Marie SK, et al. Molecular basis of infantile reversible cytochrome c oxidase deficiency myopathy. Brain. 2009;132:3165–74. doi: 10.1093/brain/awp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Raaij MJ, Abrahams JP, Leslie AG, Walker JE. The structure of bovine F1-ATPase complexed with the antibiotic inhibitor aurovertin B. Proc Natl Acad Sci USA. 1996;93:6913–7. doi: 10.1073/pnas.93.14.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonckheere AI, Renkema GH, Bras M, van den Heuvel LP, Hoischen A, Gilissen C, et al. A complex V ATP5A1 defect causes fatal neonatal mitochondrial encephalopathy. Brain. 2013;136:1544–54. doi: 10.1093/brain/awt086. [DOI] [PubMed] [Google Scholar]
- 8.Oláhová M, Yoon WH, Thompson K, Jangam S, Fernandez L, Davidson JM, et al. Biallelic mutations in ATP5F1D, which encodes a subunit of ATP synthase, cause a metabolic disorder. Am J Hum Genet. 2018;102:494–504. doi: 10.1016/j.ajhg.2018.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayr JA, Havlícková V, Zimmermann F, Magler I, Kaplanová V, Jesina P, et al. Mitochondrial ATP synthase deficiency due to a mutation in the ATP5E gene for the F1 epsilon subunit. Hum Mol Genet. 2010;19:3430–9. doi: 10.1093/hmg/ddq254. [DOI] [PubMed] [Google Scholar]
- 10.Cízková A, Stránecký V, Mayr JA, Tesarová M, Havlícková V, Paul J, et al. TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy. Nat Genet. 2008;40:1288–90. doi: 10.1038/ng.246. [DOI] [PubMed] [Google Scholar]
- 11.De Meirleir L, Seneca S, Lissens W, De Clercq I, Eyskens F, Gerlo E, et al. Respiratory chain complex V deficiency due to a mutation in the assembly gene ATP12. J Med Genet. 2004;41:120–4. doi: 10.1136/jmg.2003.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt IJ, Harding AE, Petty RK, Morgan-Hughes JA. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990;46:428–333. [PMC free article] [PubMed] [Google Scholar]
- 13.Jonckheere AI, Hogeveen M, Nijtmans LG, van den Brand MA, Janssen AJ, Diepstra JH, et al. A novel mitochondrial ATP8 gene mutation in a patient with apical hypertrophic cardiomyopathy and neuropathy. J Med Genet. 2008;45:129–33. doi: 10.1136/jmg.2007.052084. [DOI] [PubMed] [Google Scholar]
- 14.Hathazi D, Griffin H, Jennings MJ, Giunta M, Powell C, Pearce SF, et al. Metabolic shift underlies recovery in reversible infantile respiratory chain deficiency. EMBO J. 2020;39:e105364. doi: 10.15252/embj.2020105364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baran AA, Silverman KA, Zeskand J, Koratkar R, Palmer A, McCullen K, et al. The modifier of Min 2 (Mom2) locus: embryonic lethality of a mutation in the Atp5a1 gene suggests a novel mechanism of polyp suppression. Genome Res. 2007;17:566–76. doi: 10.1101/gr.6089707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE, the Mouse Genome Database Group. Mouse genome database (MGD) 2019. Nucleic Acids Res. 2019;47:D801–D806. doi: 10.1093/nar/gky1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson K, Majd H, Dallabona C, Reinson K, King MS, Alston CL, et al. Recurrent de novo dominant mutations in SLC25A4 cause severe early-onset mitochondrial disease and loss of mitochondrial DNA copy number. Am J Hum Genet. 2016;99:860–76. doi: 10.1016/j.ajhg.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gu J, Zhang L, Zong S, Guo R, Liu T, Yi J, et al. Cryo-EM structure of the mammalian ATP synthase tetramer bound with inhibitory protein IF1. Science. 2019;364:1068–75. doi: 10.1126/science.aaw4852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files. Genetic variant information is accessible in ClinVar with variation ID 432972.