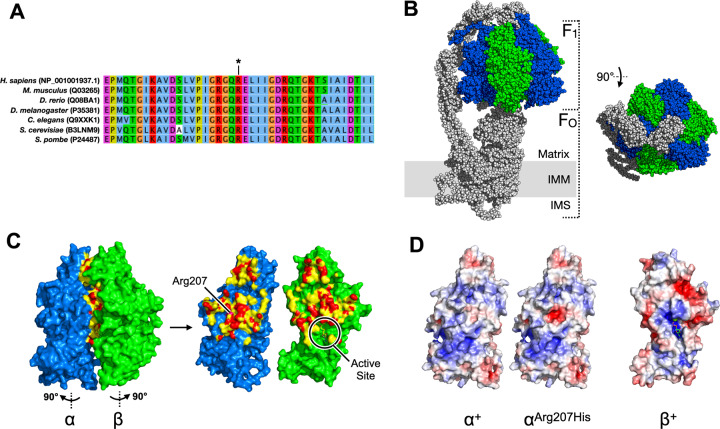
Fig. 2. Predicted functional consequence of p.Arg207His substitution in ATP5F1A.
The F1 catalytic core of complex V is a radial hexamer of alternating α and β subunits (α3β3) surrounding a central rotor (γδε). The three ATP-producing catalytic active sites lie 120° apart on each of the three β subunits, in clefts adjoining the β-α subunit interfaces. a Peptide sequences surrounding Arg207 (*) are highly conserved. b Cryo-EM structure of complex V (PDB: 6J5J) (ref. [18].). ATP5F1A (α) is shown in blue, and ATP51B (β) in green. c Detail of α-β subunit interface, per PDB:1COW (ref. [6].). Residues shown in red and yellow are within 3.6 Å and 5 Å, (respectively) of the opposing subunit. The β-subunit ATPase active site lies indirectly opposite α-Arg207 at the position shown. d at mitochondrial matrix pH (7.8), the β-interacting surface of αArg207His is predicted to display a region of central negative charge (red patch) not present in the unsubstituted protein.