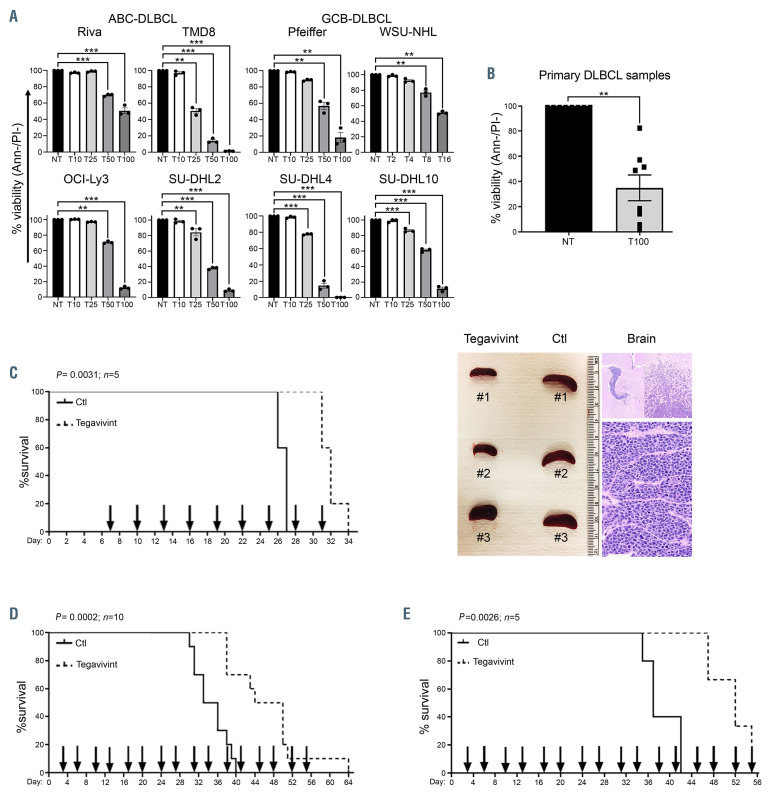
Figure 3.
Tegavivint shows significant activity in in vitro and in vivo models of diffuse large B-cell lymphoma. (A-B) Cytotoxicity assay in the indicated diffuse large B-cell lymphoma (DLBCL) cell lines (n=3) and DLBCL patient samples (n=8: peripheral blood [n=4], lymph node [n=3] and bone marrow [n=1]) treated with the indicated concentration of tegavivint (T) in nM for 24 hours. Viability was determined by annexinV/propidium (Ann-/PI-) staining and flow cytometry. Data represent means ± standard error of the mean. *P<0.05, **P<0.005, ***P<0.0005 by linear mixed effects models with adjustment for multiple dose comparisons in (A) and with two sample t-test in (B). (C) Kaplan–Meier curve showing overall survival (OS) of NSG mice engrafted with Riva (activated B-cell [ABC]-DLBCL) and randomized to receive either vehicle control (n=5) or tegavivint (n=5) given at 25 mg/kg via tail vein injection every 3 days starting at day 7 post engraftment. Median OS was 27 days for the controls (Ctl) versus 33 days for the treated group (P=0.0031 using the log-rank test). Representative picture of the spleen size (left image) and hematoxylin and eosin stain showing brain involvement by DLBCL cells (right image). (D) Kaplan–Meier curve showing OS of NSG mice engrafted with SU-DHL10 (germinal center B-cell [GCB]-DLBCL) and randomized to receive either vehicle control (n=10) or tegavivint (n=10) given at 25 mg/kg via tail vein injection twice weekly starting at day 3 post engraftment. Median OS was 34.5 days for the controls (Ctl) versus 47 days for the treated group (P=0.0002 using the log-rank test). (E) Using an adaptive transfer model of DFBL-18689, recipient NSG mice (n=5/group) were randomized to receive either vehicle control or tegavivint at 25 mg/kg via tail vein injection on a twice weekly schedule (Monday-Thursday) starting at day 3 post engraftment. Kaplan–Meier curve showing OS (P=0.0026 using the logrank test). Median OS: 55 days (tegavivint) and 37 days (control [Ctl]). Two mice in the treated group were censored due to engraftment failure assessed by flow cytometry for circulating lymphoma cells and magnetic resonance imaging for spleen volume.