Figure 5. REV3L localizes in heterochromatin through a direct interaction with HP1 dimer.
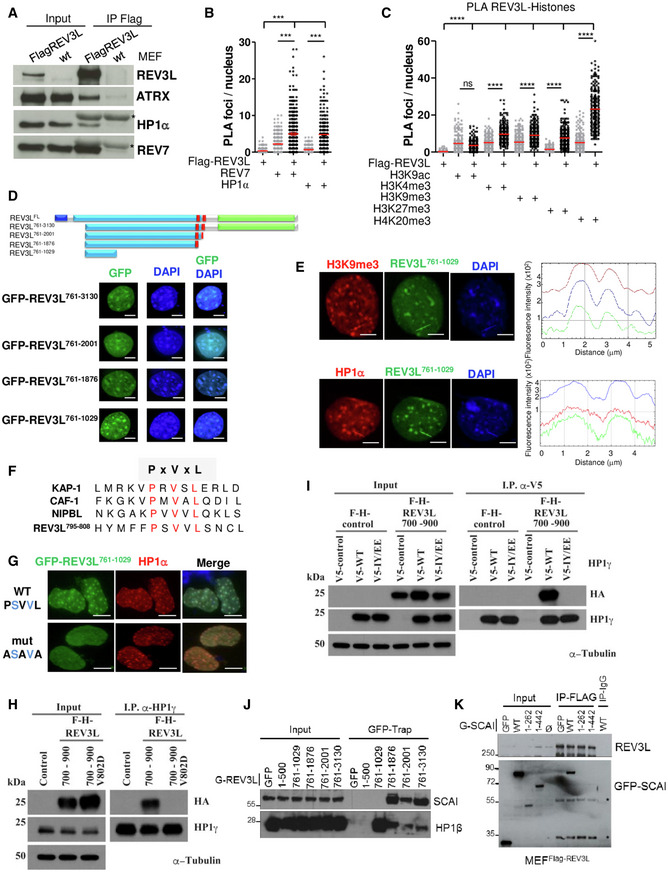
- Asynchronous MEFs expressing Flag‐tagged REV3L from the endogenous locus were lysed, and REV3L was immunoprecipitated using anti‐Flag (M2) antibodies. Co‐immunoprecipitated proteins were analyzed by immunoblotting using the indicated antibodies. *: IgG light chain. This experiment was repeated 2–4 times.
- Asynchronous MEFs expressing Flag‐tagged REV3L were subjected to in situ proximity ligation assay (PLA) to test the interactions REV3L‐REV7 and REV3L‐HP1α. Nuclear foci were quantified (more than 150 nuclei for each condition were counted). Reactions omitting one of the primary antibodies were used as negative controls. Horizontal bars show the mean. Mann–Whitney test, ns: not significant, ***P < 0.001. Experiments were repeated three times.
- Asynchronous MEFs expressing Flag‐tagged REV3L were subjected to PLA to test the interactions REV3L‐H4K20me3, REV3L‐H3K9me3, REV3L‐H3K27me3, REV3L‐H3K4me3, and REV3L‐H3K9ac. Nuclear foci were quantified as in (B). Horizontal bars show the mean. Mann–Whitney test, ns: not significant, ****P < 0.0001. Three independent experiments were performed.
- Schematic representation of human REV3L and truncated constructs. Conserved domains between yeast and human REV3L are in black blue (the N‐terminal domain, 1–333 aa) and in green (the catalytic domain, 2,276–3,130 aa), the REV7 interacting domains are in red (1,880–2,001 aa). A large region in royal blue not found in yeast protein is almost exclusively encoded by exon 14. All the truncated constructs lack the NTD domain and were fused to GFP. MEFs cells were transiently transfected with various GFP‐REV3L constructs and fixed with 4% formaldehyde. The distribution of the GFP‐REV3L mutants was detected by autofluorescence, and nuclei were visualized using DAPI staining. Scale bar = 5 μm.
- MEFs cells were transiently transfected with GFP‐REV3L761–1,029 and fixed with 4% formaldehyde. The distribution of GFP‐ REV3L761–1,029 was detected by autofluorescence (green), chromocenters were visualized by H3K9me3 immunostaining (red, top panel) or HP1α (red, bottom panel), and DNA was counterstained with DAPI. Line scans represent the colocalization of proteins within each image (right panels). Scale bar = 5 μm.
- Sequence alignment of proteins containing the PxVxL motif important for an interaction with HP1, with canonical residues shown in red.
- MEFs cells were transfected with GFP‐REV3L761–1,029 WT PSVVL or ASAVA mutant and fixed with 4% formaldehyde. The distribution of GFP‐REV3L constructs was detected by autofluorescence (green), chromocenters were visualized by HP1α immunostaining (red), and DNA was counterstained with DAPI. Scale bar = 10 μm.
- Human 293T cells were transfected with either FH‐REV3L700–900, mutant V802D F‐H‐REV3L700–900, or empty vector. Forty‐eight hours after transfection, cell lysates were made and used for immunoprecipitation with HP1 antibody. Western blot was processed, and membranes were immunoblotted with the indicated antibodies.
- 293T cells were co‐transfected with F‐H‐REV3L700–900 or empty vector and V5‐HP1γ, mutant IY/EE V5‐HP1γ or empty vector. Forty‐eight hours after transfection, cell lysates were made and used for immunoprecipitation with α‐V5 antibody. After electrophoresis, samples were immunoblotted with anti‐HA, anti‐HP1γ, or anti‐α‐Tubulin as indicated.
- 293 cells were transfected with various GFP‐REV3L constructs or empty vector (GFP). Twenty‐four hours after transfection, cell lysates were made and GFP‐REV3L was affinity‐purified on GFP‐Trap beads. After electrophoresis, samples were analyzed by immunoblotting with antibodies against SCAI or HP1β as indicated.
- MEFFlag‐REV3L were transfected with various GFP‐SCAI constructs, empty vector (GFP), or not transfected (Ø). Twenty‐four hours after transfection, cell lysates were made and Flag‐REV3L was affinity‐purified on M2 beads. After electrophoresis, samples were analyzed by immunoblotting with antibodies against GFP or Flag (M2). *: non‐specific bands.
Source data are available online for this figure.
