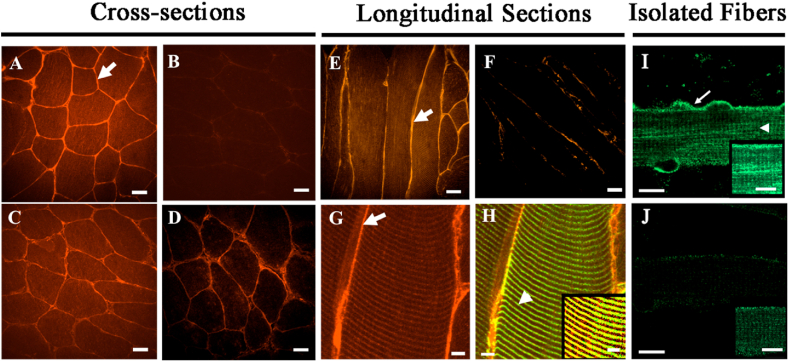
Fig. 2.
Immunolabeling of μ-crystallin in control and Crym tg skeletal muscles. A-D, Cross (A–D), longitudinal sections (E–H), of Crym tg (A, C, E, G, H) and control (B, D, F) TA (A, B, E-H) and soleus muscles (C, D) were stained with anti-μ-crystallin antibody and Alexa Fluor-568-conjugated secondary antibody. In G-H, longitudinal sections of Crym tg TA muscle (G, μ-crystallin only) were colabeled with anti-desmin and Alexa Fluor-488-conjugated secondary antibody (H, yellow color shows colabeled structures). I, J, Flexor digitorum brevis (FDB) myofibers in culture from Crym tg (I) and control mice (J) were labeled with anti-μ-crystallin antibody and Alexa-Fluor 488-conjugated secondary antibody. The results show that μ-crystallin was detected at higher levels in tg muscles than in controls. In TA muscle and FDB myofibers it was enriched at the levels of the sarcolemma (arrows, A,E,G, I) and Z-disks, colabeled with desmin (arrowhead, H) or shown without desmin colabel (arrowhead, I). Inset panels (H–J) are brightened and magnified. Crym tg (I) and control (J) FDB images were brightened and magnified equivalently. A-F, scale bars = 20 μm; G, H, scale bars = 5 μm; I, J, scale bars = 10 μm.