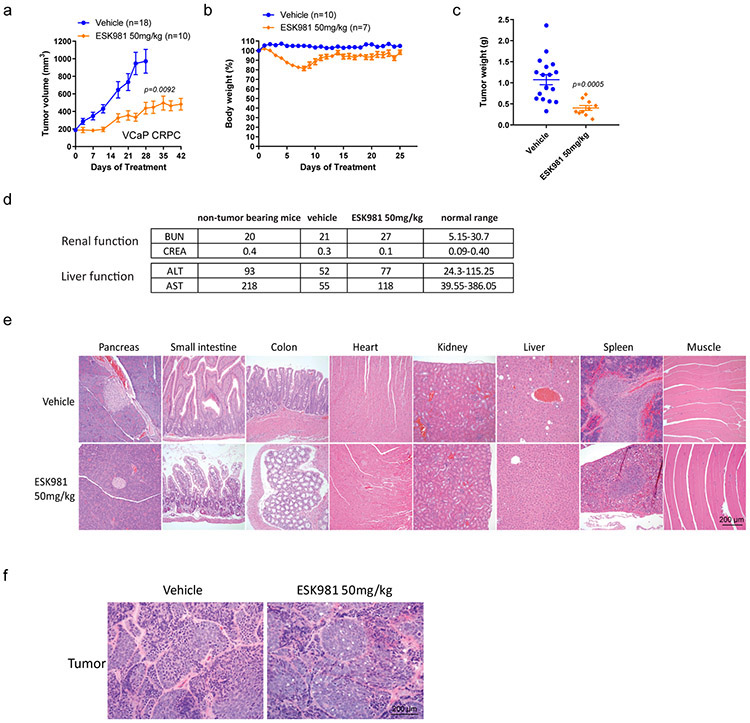
Extended Data Fig. 3. Renal function, liver function, and histopathological evaluation of ESK981-treated xenografts.
(a) Castration-resistant VCaP tumors were established according to Extended Data Fig. 2a. Tumor-bearing mice were divided into vehicle and ESK981 50 mg/kg groups, and tumor volumes were monitored twice per week for six weeks. Data were analyzed by two-tailed unpaired t test and presented as mean ± SEM at day 25. N=number of tumors and P-value indicated.
(b) The percent body weights of VCaP tumor-bearing mice were monitored daily throughout this study. Data were presented as mean ± SEM. N=number of mice.
(c) The weight of VCaP tumors from vehicle (n=18 tumors) and ESK981 50 mg/kg (n=10 tumors) were measured at the end of this study. Data were analyzed by two-tailed unpaired t test and presented as mean ± SEM. P-value indicated.
(d) Blood chemistry was evaluated for renal and liver functions in non-tumor-bearing and VCaP tumor-bearing mice in vehicle and 50 mg/kg ESK981 treatment groups.
(e) Representative histological sections showing H&E staining for various organs taken from vehicle- or ESK981-treated mice from three independent experiments.
(f) Representative histological sections showing H&E staining for tumors taken from vehicle- or ESK981-treated mice from three independent experiments.