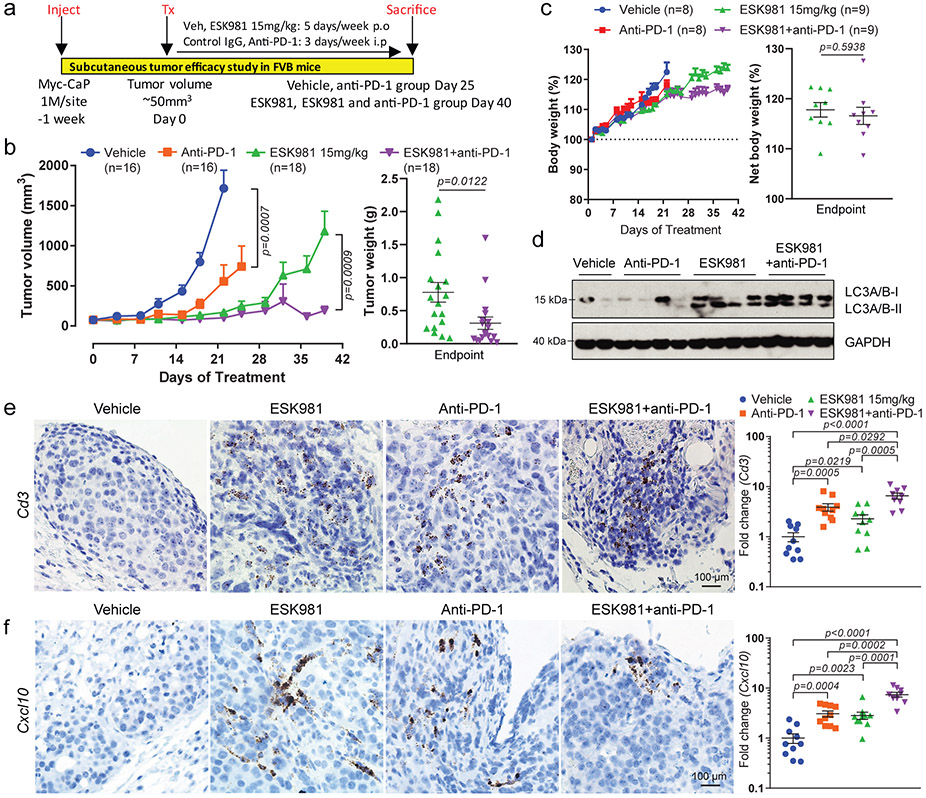
Fig. 6. ESK981 potentiates the effect of anti-PD-1 immunotherapy in immune-competent murine prostate cancer models.
(a) Illustration of Myc-CaP experimental design in immune-competent FVB mice. p.o, oral gavage. i.p, intraperitoneal.
(b) Average Myc-CaP tumor volumes from indicated treatment (left). Myc-CaP tumor weights were measured at endpoint from ESK981 and ESK981 plus anti-PD-1 (right). Data were analyzed by two-tailed unpaired t test and presented as mean ± SEM. N=number of tumors, and P-values are indicated.
(c) Percent body weight changes of Myc-CaP tumor-bearing mice receiving indicated treatment (left); data were presented as mean ± SEM. Net body weight changes were calculated by exclusion of tumor weight from total body weight in ESK981 and ESK981+anti-PD-1 treated mice at day 40 (right); data were analyzed by two-tailed unpaired t test and presented as mean ± SEM. N=number of mice, and P-value is indicated.
(d) Protein levels of LC3 from representative individual tumors measured by western blot after five days of the indicated treatment in Myc-CaP tumors. N=2 for vehicle; n=4 for anti-PD-1; n=4 for ESK981; n=4 for ESK981+anti-PD-1 with three independent experiments.
(e) Representative Cd3 RNA ISH from the indicated Myc-CaP tumors with three independent experiments (left). For a complementary method of assessing Cd3 levels, Cd3 mRNA levels were quantified by qPCR in individual tumors treated with vehicle (n=10 tumors), ESK981 15 mg/kg (n=10 tumors), anti-PD-1 (n=10 tumors), ESK981 and anti-PD-1 (n=9 tumors) (right). Data from vehicle and ESK981 15 mg/kg tumors were included for different comparison purposes in Fig. 5e. Data were analyzed by two-tailed unpaired t test and presented as mean ± SEM. P-value indicated.
(f) Representative Cxcl10 RNA ISH from the indicated Myc-CaP tumors with three independent experiments (left). For a complementary method of assessing Cxcl10 levels, Cxcl10 mRNA levels were quantified by qPCR in individual tumors treated with vehicle (n=10 tumors), ESK981 15 mg/kg (n=10 tumors), anti-PD-1 (n=10 tumors), ESK981 and anti-PD-1 (n=9 tumors), after three weeks of the indicated treatments in Myc-CaP tumors (right). Data from vehicle and ESK981 15 mg/kg tumors were included for different comparison purposes in Fig. 5e. Data were analyzed by two-tailed unpaired t test and presented as mean ± SEM. P-value indicated.