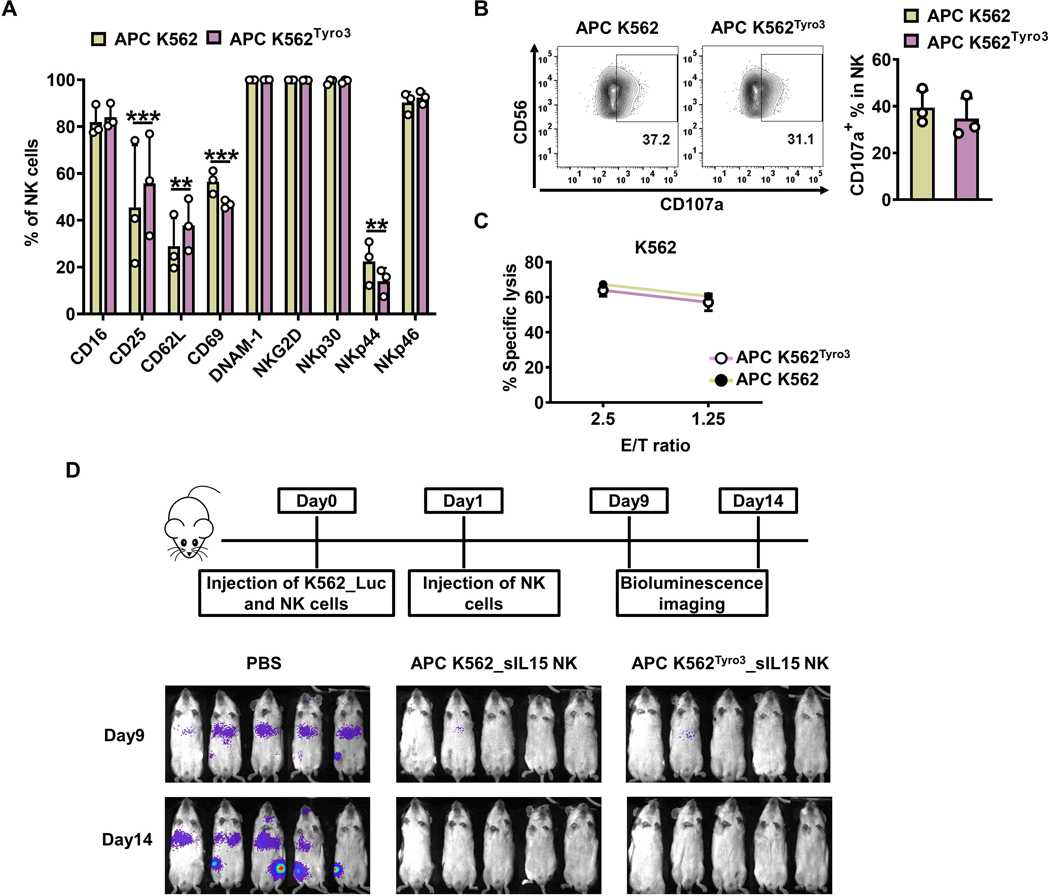
Figure 6. TYRO3-expressing K562 feeder cells expand NK cells with similar in vivo antitumor activity.
(A-C) Primary human NK cells were incubated with inactivated APC K562 cells or APC K562Tyro3 cells in the presence of IL2 (50 IU/ml) for 7 days. Summary data from 3 different donors showing the expression of surface markers on NK cells expanded with APC K562 cells or APC K562Tyro3 cells (A). Representative flow cytometry plots and summary data from 3 different donors showing the expression of CD107a on NK cells expanded with APC K562 cells or APC K562Tyro3 cells for 4 h at an E/T ratio of 4:1 (B). NK cells expanded with APC K562 cells or APC K562Tyro3 cells were co-cultured with 51Cr-labeled K562 cell line for 4 h, followed by quantification of specific K562 cell lysis. Data are summarized from 3 different donors (C). (D) Primary human NK cells were incubated with APC K562Tyro3 or APC K562 cells in the presence of IL2 (50 IU/ml) for 4 days. Then cells were transduced with a retrovirus vector expressing soluble IL15 (sIL15) for 48 h. On day 6, transduced NK cells were stimulated with APC K562Tyro3 cells or APC K562 cells in the presence of IL2 (50 IU/ml) for another 7 days prior to harvest and storage for following usage. On day 0, mice were injected with 1×106 K562_Luc cells and then treated with 10×106 sIL15 NK cells expanded with APC K562 (APC K562_sIL15 NK) or APC K562Tyro3 (APC K562Tyro3_sIL15 NK). On day 1, mice were treated with a 2nd dose of these NK cells. Bioluminescence imaging was performed on days 9 and 14. Five mice in each group. Two-way ANOVA was used for A. **, P<0.01; ***, P<0.001. Data are presented as mean ± SD.