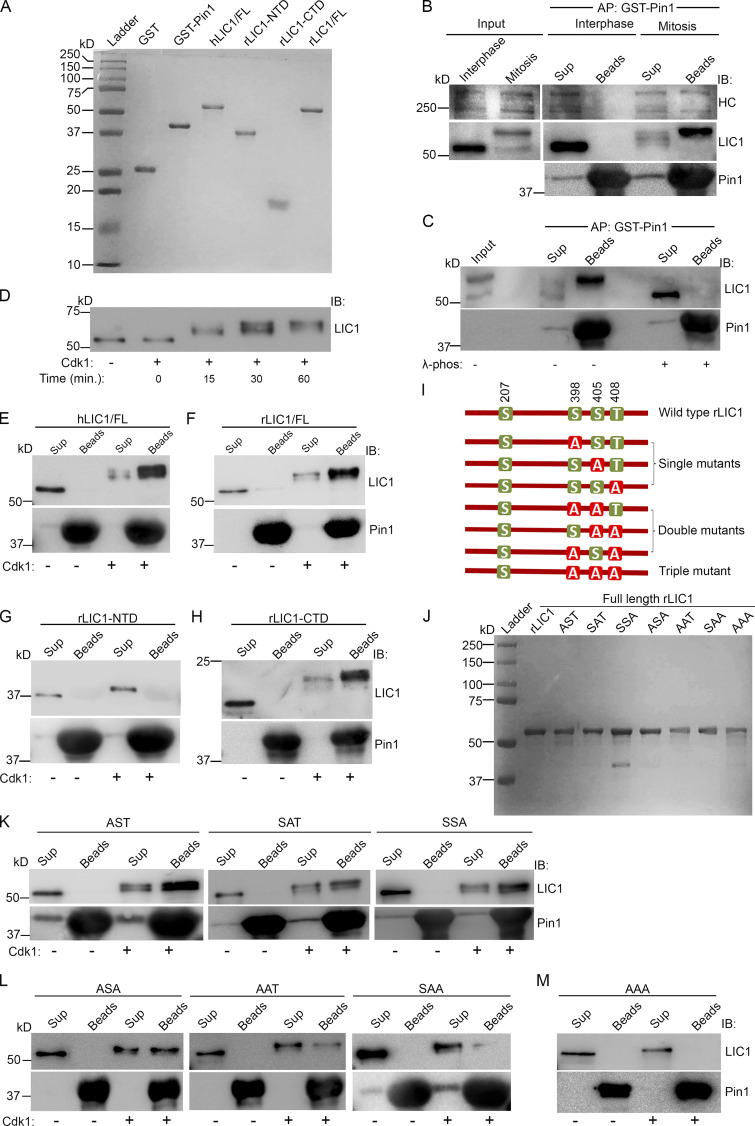
Figure 3.
LIC1-CTD phosphorylation is required for Pin1 to interact with mitotic dynein. (A) Coomassie-stained SDS-PAGE (15%) of the indicated recombinantly purified proteins. (B) Immunoblots showing the binding of mitotic dynein, but not interphase dynein, from HeLa cell lysates with purified GST-Pin1. (C) Immunoblots of the affinity precipitates from A after λ-phosphatase (λ-phos) treatment. (D) Anti-LIC1 immunoblot of purified hLIC1 after incubation with purified cdk1-cyclin B for the indicated durations. (E–H) Immunoblots showing the direct binding of purified hLIC1 (E), rLIC1 (F), and rLIC1-CTD (H),but not rLIC1-NTD (G) with purified GST-Pin1 in the presence (+) or absence (–) of cdk1. (I and J) Schematic diagram (I) and Coomassie-stained SDS PAGE (10%) profiles (J) of the indicated purified proteins. (K–M) Immunoblots depicting direct binding assays of the indicated purified rLIC1 proteins with purified GST-Pin1, with (+) and without (–) prior cdk1 treatment. AP, affinity purification; IB, immunoblot, Cdk1, cdk1-cyclin B; beads, affinity precipitate; sup, supernatant.