Abstract
BACKGROUND:
Twin-twin transfusion syndrome presents many challenges for clinicians, and the optimal means of identifying pregnancies that will benefit most from intervention is controversial. There is currently no clinically available biomarker to detect twin-twin transfusion syndrome or to stratify cases based on the risk factors. microRNAs are small RNAs that regulate gene expression and are biomarkers for various disease processes, including adult and pediatric heart failure. To date, no studies have investigated amniotic fluid microRNAs as biomarkers for disease severity, specifically for severe recipient cardiomyopathy in twin-twin transfusion syndrome cases.
OBJECTIVE:
This study aimed to assess whether amniotic fluid microRNAs could be useful as biomarkers to identify pregnancies at greatest risk for severe recipient cardiomyopathy associated with twin-twin transfusion syndrome.
STUDY DESIGN:
Amniotic fluid was collected at the time of amnioreduction or selective fetoscopic laser photocoagulation from monochorionic diamniotic twin pregnancies with twin-twin transfusion syndrome at any stage. Fetal echocardiography was performed on all twins before the procedure, and severe cardiomyopathy was defined as a right ventricular myocardial performance index of the recipient fetus of >4 Z-scores. microRNA was extracted from the amniotic fluid samples and analyzed using an array panel assessing 379 microRNAs (TaqMan Open Array, ThermoFisher). Student t tests were performed to determine significant differences in microRNA expression between pregnancies with severe recipient cardiomyopathy and those with preserved cardiac function. A stringent q value of <.0025 was used to determine differential microRNA expression. Random forest plots identified the top 3 microRNAs that separated the 2 groups, and hierarchical cluster analysis was used to determine if these microRNAs properly segregated the samples according to their clinical groups.
RESULTS:
A total of 14 amniotic fluid samples from pregnancies with twin-twin transfusion syndrome with severe cardiomyopathy were compared with samples from 12 twin-twin transfusion syndrome control cases with preserved cardiac function. A total of 110 microRNAs were identified in the amniotic fluid samples. Twenty microRNAs were differentially expressed, and the top 3 differentiating microRNAs were hsa-miR-200c-3p, hsa-miR-17-5p, and hsa-miR-539-5p. Hierarchical cluster analysis based on these top 3 microRNAs showed a strong ability to differentiate severe cardiomyopathy cases from controls. The top 3 microRNAs were used to investigate the sensitivity and specificity of these microRNAs to differentiate between the 2 groups with a receiver operating characteristic curve demonstrating sensitivity and specificity of 80.8%. All 20 differentially expressed microRNAs were down-regulated in the group with severe cardiomyopathy.
CONCLUSION:
Amniotic fluid microRNAs demonstrated differential expression between twin-twin transfusion syndrome recipient fetuses with severe cardiomyopathy and those without and have the potential to be important biomarkers of disease severity in this population.
Keywords: biomarker, heart failure, monochorionic, myocardial performance index, selective fetoscopic laser photocoagulation, Tei index, ultrasound
Introduction
Twin-twin transfusion syndrome (TTTS) affects 10% to 15% of monochorionic diamniotic (MCDA) twin pregnancies and is a major source of neonatal morbidity and mortality, accounting for 15% of all twin perinatal mortalities and up to half of MCDA perinatal deaths.1–3 Disease severity is initially determined by the Quintero staging system4 with TTTS beyond stage III being associated with a high morbidity and mortality risk for both twins.2 Three-quarters of Quintero stage I TTTS cases remain stable or the condition resolves, but a limitation in the staging of TTTS is that disease progression does not correlate well with the initial stage of disease.2 Thus, the current recommendations are to perform serial ultrasounds beginning at 16 weeks’ gestation to screen for TTTS, which most often presents in the second trimester.2
TTTS is caused by an imbalance in blood volume and vasoactive mediators between the 2 fetuses owing to vascular connections to a single placenta.2,5 The resulting volume depletion in the donor twin and volume overload in the recipient twin leads to the disease spectrum called TTTS. In addition, studies suggest that the renin-angiotensin-aldosterone system (RAAS) is activated in the donor twin owing to hypovolemia, and RAAS components are transferred to the recipient twin via the placental anastomoses, perpetuating a vicious cycle of imbalance that potentially contributes to the development of cardiomyopathy in the recipient twin.6
The development of cardiomyopathy in the recipient twin is associated with increased mortality than in recipients with preserved cardiac function.7,8 Cardiomyopathy may lead to right ventricular outflow tract obstruction over time, further increasing morbidity.9 Because of the prevalence and worsened outcomes of recipient cardiomyopathy in TTTS, many centers include fetal echocardiography in their evaluation of TTTS and in counseling regarding treatment options.5,10–14 The Cincinnati modification of the Quintero staging system12 is an alternative staging system that has been proposed and includes the severity of cardiomyopathy in the recipient fetus based on atrioventricular valve (AV) regurgitation, ventricular hypertrophy, and the myocardial performance index (MPI) as detailed by Habli et al.5 The MPI is a Doppler index of ventricular dysfunction first described by Tei et al15 as the ratio of ventricular isovolumetric contraction and relaxation time to ventricular ejection time, with a higher value representing more severe dysfunction.5,16 As described by Habli et al,5 MPI Z-scores of >+2, +3, and +4 correspond to mild, moderate, and severe cardiomyopathy, respectively. Ventricular hypertrophy is assessed qualitatively as mild, moderate, or severe, and AV regurgitation is semi-quantitatively assessed as mild, moderate, or severe based on the relative size of the regurgitant jet on Doppler interrogation.10 In recipient cardiomyopathy, MPI dysfunction typically precedes abnormalities in ventricular hypertrophy or AV regurgitation, and the right ventricular (RV) MPI has a high likelihood of correction after laser treatment to ablate the vascular anastomoses between placental shares.10,14 However, timing the laser therapy in TTTS cases in which disease progression is not predictable remains a challenge. Thus, several studies have attempted to identify biomarkers for TTTS using maternal serum and amniotic fluid17–19; however, no clinically significant biomarkers have been identified to date. Recently, microRNAs (miRNAs) have been reported as biomarkers for various cardiovascular conditions, but there have been limited investigations in pregnancy and fetal disease.20,21
miRNAs are small, noncoding RNAs involved in regulation of gene expression and stress signaling pathways and have demonstrated usefulness as biomarkers for various disease states.22–25 miRNAs are present in multiple body fluids, including amniotic fluid, with distinct patterns of expression under normal and pathologic conditions.26 In urine and serum, miRNAs have shown promise as biomarkers for cancers and cardiovascular diseases in children and adults.23,24,26,27 Differential expression of miRNAs has been identified in the failing heart, both in adult and pediatric cardiomyopathy, and miRNAs may play a direct role in cardiac remodeling.22 Specific miRNAs detected in serum correlate with worsened outcomes in children with dilated cardiomyopathy,25 and myocardial miRNA profiles in hypoplastic left heart syndrome have been shown to differ from those of normal hearts, with changes in expression after surgical intervention.28 Furthermore, miRNAs have unique potential as future therapeutic targets owing to their effect on messenger RNA (mRNA) transcription.29
There is emerging evidence to support that amniotic fluid miRNAs play a role in normal fetal development and that specific miRNA profiles may have a prognostic role in fetal diseases.20,30–32 Amniotic fluid miRNAs have not been studied previously in TTTS as a biomarker for cardiomyopathy.
Study objective
The objective of this study was to profile amniotic fluid miRNA expression in pregnancies with TTTS with and without severe cardiomyopathy. Our hypothesis was that pregnancies affected by TTTS with severe cardiomyopathy would have substantially different amniotic fluid miRNA profiles than TTTS pregnancies with preserved cardiac function.
Materials and Methods
Patients were eligible for inclusion if they had an MCDA twin gestation with sonographic evidence of TTTS at any stage or gestational age and if they underwent either amnioreduction or selective fetoscopic laser photocoagulation (SFLP) at a single, high-volume fetal center (Colorado Fetal Care Center, Children’s Hospital Colorado, Aurora, CO). Pregnancies without fetal echocardiography or clinical outcome data were excluded. All fetal echocardiography procedures were performed by experienced fetal cardiac sonographers and were interpreted by a pediatric cardiologist. Severe cardiomyopathy was defined as an RV MPI Z-score of >4 or ≥0.64 for the Cincinnati modification of the Quintero staging system.5 Maternal demographic information and clinical data were obtained by chart review and stored in the REDCap system.
A sample size of 12 per group was targeted based on preliminary data demonstrating differential miRNA expression in the amniotic fluid of singletons and MCDA twins with TTTS33 and estimating that a 20% greater sample size would be needed to account for the anticipated sample similarity when using MCDA twin controls.
Amniotic fluid was collected from the recipient sac at the time of amnioreduction or SFLP at the time of initial entry into the recipient sac and before laser therapy. Whole amniotic fluid was centrifuged at 900× g for 10 minutes before freezing at −80°C. miRNA was extracted from the supernatant of each sample using the Qiagen miRNeasy Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. The TaqMan Open Array miRNA panel (ThermoFisher Bedford, MA) was then used to assess the expression profile of 379 miRNAs.
Array data were analyzed using Expression Suite Software (ThermoFisher, Waltham, MA). The expression levels for all miRNAs were normalized to the expression level of hsa-miR-363, because this miRNA showed minimal variability in expression levels among all samples, including a preliminary cohort of singletons (not shown).33 To remove any potential batch effect from separately run samples, miRNA arrays were repeated for 1 severe cardiomyopathy sample to evaluate consistency among batches. The values were normalized between runs and values from the repeated sample were averaged. miRNAs were considered expressed in amniotic fluid if they were detected in at least 80% of the samples and had a cycle threshold <32. Statistical analysis of the miRNA data was performed using R (www.R-project.org) and a heat map was plotted using the heatmap.2 function in the gplots package in R. Student t tests of the log2 transformed data were performed for comparison among groups and a stringent q value of <.0025 (Wilcoxon) was used to define differentially expressed miRNAs while accounting for multiple comparisons. Random forest (RF) analysis using 50,000 trees in R was performed to classify the groups based on miRNA expression. The top 3 miRNAs that differentiated the severe cardiomyopathy group from the controls were used for all subsequent analyses, including hierarchical clustering and receiver operating characteristic (ROC) curves. RF analysis uses 2 methods for measuring the significance of a variable and how much it contributes to an accurate prediction, namely mean decrease accuracy and mean decrease Gini. The area under the ROC curve was calculated using the pROC package in R. ROC curves were calculated to determine the sensitivity and specificity for the top 3 miRNAs. Clinical variables and outcome data were compared using Student t tests for continuous variables and Fisher exact tests for categorical variables, and significance was set a priori at P<.05.
This study was a secondary analysis of amniotic fluid obtained with informed consent for the study of biomarkers in TTTS (COMIRB Protocol 14-1413; principal investigator, B.F.C.). This study was reviewed by the Colorado Multiple Institution Review Board and was exempt as a secondary analysis (COMIRB Protocol 19-1764; principal investigator, E.C.W.).
Results
Amniotic fluid samples from 14 pregnancies with TTTS with severe cardiomyopathy were compared against samples from 12 pregnancies with TTTS with preserved cardiac function. Maternal demographics and prenatal characteristics are presented in Table 1. There was no difference among the groups with regard to maternal age, body mass index (BMI), gestational age at procedure, fetal sex, presence of selective intrauterine growth restriction (estimated weight <10th percentile) of the donor twin, Quintero stage, or type of procedure performed at the time of fluid collection (all P values >.05). Recipient cardiovascular function differed significantly between the 2 groups with respect to RV MPI, left ventricular MPI, ventricular hypertrophy, and AV regurgitation (all P values <.01). The clinical outcome data are presented in Table 2. There was no significant difference among groups with respect to gestational age at delivery, latency from procedure to delivery, or neonatal survival (all P values >.05).
TABLE 1.
Maternal demographics and pregnancy characteristics
| Mean (SD) | Mean (SD) | ||
|---|---|---|---|
| Maternal age (y) | 28.3 (4.1) | 27.8 (5.8) | .82 |
| BMI (kg/m2) | 28.4 (4.2) | 28.6 (5.2) | .92 |
| GA at procedure (wk) | 20.3 (2.8) | 19.5 (2.0) | .38 |
| Recipient preprocedure RV MPI | 0.99 (0.39) | 0.40 (0.07) | <.01 |
| Recipient preprocedure LV MPI | 0.70 (0.25) | 0.39 (0.04) | <.01 |
| n (%) | n (%) | ||
| Quintero stage | .48 | ||
| I | 1 (7) | 2 (17) | |
| II | 4 (29) | 1 (8) | |
| III | 8 (57) | 9 (75) | |
| IV | 1 (7) | 0 (0) | |
| Ventricular hypertrophy | <.01 | ||
| None | 1 (7) | 8 (67) | |
| Mild | 5 (36) | 4 (33) | |
| Moderate | 6 (43) | 0 (0) | |
| Severe | 2 (14) | 0 (0) | |
| AV regurgitation | <.01 | ||
| None | 4 (29) | 11 (92) | |
| Mild | 5 (36) | 1 (8) | |
| Moderate | 4 (29) | 0 (0) | |
| Severe | 1 (7) | 0 (0) | |
| Fetal sex | 1 | ||
| Male | 8 (57) | 6 (50) | |
| Female | 6 (43) | 6 (50) | |
| sIUGR of donor | 1 | ||
| Present | 9 (64) | 8 (67) | |
| Absent | 5 (36) | 4 (33) | |
| Type of procedure | .46 | ||
| Amnioreduction | 0 (0) | 1 (8) | |
| SFLP | 14 (100) | 11 (92) |
Data are presented as mean (standard deviation) unless otherwise mentioned.
AV, atrioventricular valve; BMI, body mass index; GA, gestational age; LV, left ventricle; MPI, myocardial performance index; RV, right ventricle; SD, standard deviation; SFLP, selective fetoscopic laser photocoagulation; sIUGR, selective intrauterine growth restriction.
TABLE 2.
Pregnancy outcomes
| Mean (SD) | Mean (SD) | ||
|---|---|---|---|
| Gestational age at delivery (wk) | 28.8 (5.4) | 28.1 (4.0) | .71 |
| Latency from procedure to delivery (wk) | 8.5 (4.7) | 8.6 (4.5) | .92 |
| Latency from procedure to delivery (d) | 59.2 (32.9) | 60.4 (31.4) | .92 |
| n (%) | n (%) | ||
| Neonatal survival | .74 | ||
| Both twins | 8 (57) | 8 (67) | |
| One twin | 3 (21) | 3 (25) | |
| Neither twin | 3 (21) | 1 (8) | |
| Overall neonatal survival | 19 (67) | 19 (79) | .53 |
Data are presented as mean (standard deviation) and number (percentage) unless otherwise indicated.
A total of 110 miRNAs were identified in the amniotic fluid samples. Heat map analysis based on t test analyses demonstrated 20 differentially expressed miRNAs with a significant q value of <.0025 (Figure 1, A). A list of these 20 miRNAs is provided in Supplemental Table. RF classification identified miR-200c-3p, miR-17-5p, and miR-539-5p as the top 3 discriminators of severe cardiomyopathy cases from controls, as demonstrated by the highest mean decrease in accuracy and Gini coefficients (Figure 1, B). RF analysis identified the top 3 miRNAs that correctly classified the samples into 2 groups, as demonstrated by the separation of patients in the severe cardiomyopathy group (red triangles) from the control group patients (blue squares) (Figure 2, A). Box plots showed significantly lower expression of miR-200c-3p, miR-17-5p, and miR-539-5p in the severe cardiomyopathy group than in the controls (0.25±0.37 vs 1.19±0.82; q<0.0025; 0.31±0.45 vs 1.17±0.84; q<0.0025; 0.25±0.39 vs 1.18±0.94; q<0.0025, respectively) (Figure 2, B). Hierarchical clustering based on the top 3 miRNAs showed a strong ability to differentiate the severe cardiomyopathy group from the controls (Figure 2, C). Based on the ROC curves of the top 3 miRNAs, there is an 80.8% specificity and sensitivity to differentiate the severe cardiomyopathy group from the controls (Figure 2, D).
FIGURE 1. Heat map and random forest classification of differentially expressed microRNAs.
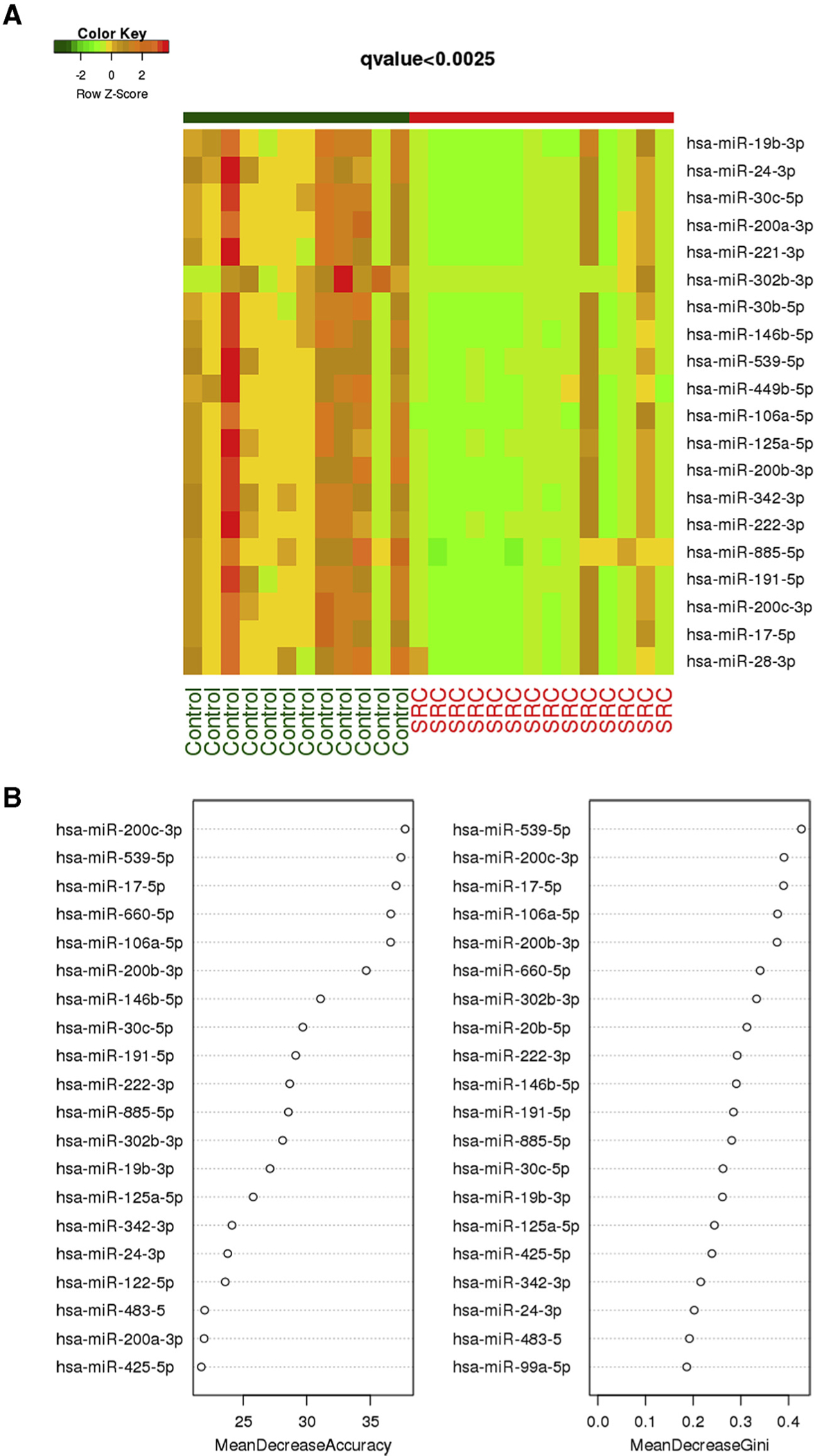
A, Heat map based on Wilcoxon q<0.0025 can separate miRNA expression levels between TTTS cases with SRC (n=14) and controls (n=12). B, Random forest classification identified miRNA-200c-3p, miR-17-5p, and miR-539-5p as the best discriminators between cases with SRC and control cases, as demonstrated by the highest mean decrease in accuracy and Gini coefficient.
miRNA, microRNA; SRC, severe recipient cardiomyopathy; TTS, twin-to-twin transfusion syndrome.
FIGURE 2. Random forest analysis of differentially expressed miRNAs.
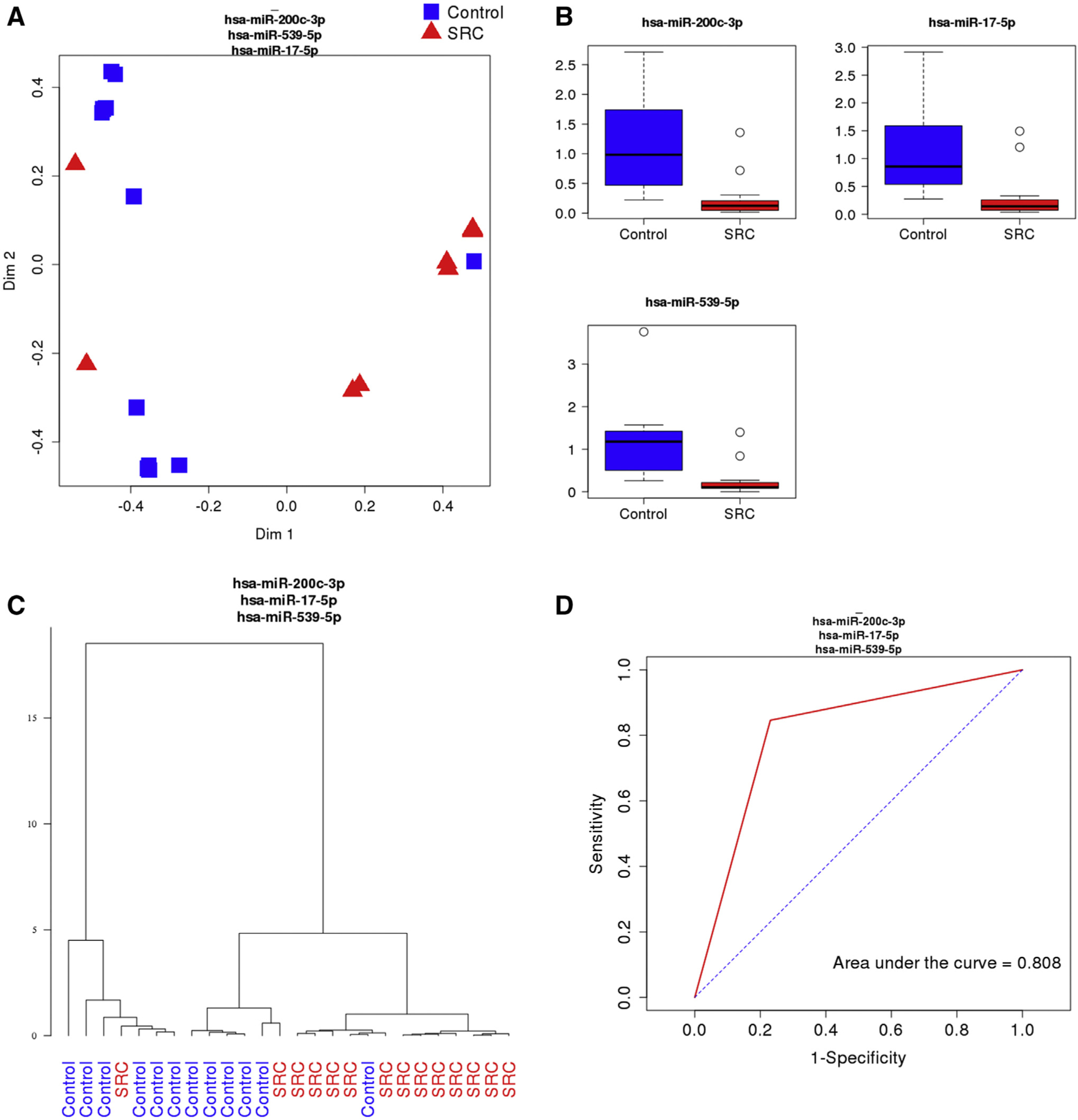
A, Random forest analysis of the top 3 miRNAs demonstrated differential expression between the SRC group (red triangles, n=14) and the control group (blue squares, n=12). B, Expression of miR-200c-3p (q=.0019), miR-17-5p (q=.0019), and miR-539-5p (q=.0023) were significantly lower in the SRC group than in the control group. C, Hierarchical clustering analysis shows a strong ability to differentiate the SRC group from the controls based on the expression levels of the top 3 miRNAs. D, Receiver operating characteristic curve of the top 3 miRNAs demonstrate an area under the curve of 0.808.
miRNA, microRNA; SRC, severe recipient cardiomyopathy; TTS, twin-to-twin transfusion syndrome.
Comment
Principal findings
We identified 20 differentially expressed miRNAs in the amniotic fluid of recipient fetuses in cases of TTTS with severe cardiomyopathy when compared with TTTS controls with preserved cardiac function. All 20 miRNAs were down-regulated in the severe cardiomyopathy group. The top 3 differentially expressed miRNAs, namely miR-200c-3p, miR-17-5p, and miR-539-5p, represent potential diagnostic biomarkers for severe recipient cardiomyopathy in TTTS cases.
Results
Our results suggested altered fetal pathophysiology in TTTS pregnancies complicated by severe recipient cardiomyopathy based on the top 3 differentially expressed miRNAs, all having potential cardiac targets.
There is evidence that miRNA-17 is cardioprotective and that it has multiple targets involved in stress adaptation,34 collagen secretion, fibroblast proliferation,35,36 and resistance to apoptosis37,38 in addition to its potential role in cardiac progenitor cell differentiation to cardiomyocytes during fetal development as part of the miRNA-17-92 cluster.39,40 miRNA-200c may also play a cardioprotective role41 and has several cardiac targets. Dysregulation of miRNA-200c in cardiomyocytes has been associated with hypoxia-induced apoptosis,42 cardiomyocyte hypertrophy,43,44 cardiomyocyte ion channel remodeling,45 and oxidative stress response.41 miRNA-539 has been shown to regulate mitochondrial fission and apoptosis46 and is dysregulated in patients with Duchenne muscular dystrophy,47 which affects both skeletal and cardiac muscles. At least 1 cardiac-specific target of miRNA-539 has been identified, and dysregulation of miRNA-539 in a rat model has been associated with heart failure.48,49
These findings are consistent with the limited previous data regarding miRNA and mRNA expression in TTTS. Mackie et al50 found differential expression of several miRNAs in maternal serum from pregnancies with TTTS when compared with healthy MCDA pregnancies; however, their study was underpowered to detect a substantial difference after controlling for multiple comparisons. Hui et al51 identified differential mRNA expression of more than 800 genes related predominantly to neurologic and cardiovascular pathways in the amniotic fluid of TTTS recipient fetuses than in singleton controls.51
Clinical implications
Not all centers utilize fetal echocardiography in the determination of TTTS severity and consensus regarding the data to support inclusion of a cardiomyopathy assessment in determining treatment candidates is lacking.2 Our study provides insight into the altered fetal pathophysiology among TTTS recipient fetuses with severe cardiomyopathy and novel miRNA-based amniotic fluid biomarkers for severe disease cases. Further investigations in larger populations are required before the value of amniotic fluid miRNA biomarkers can be fully elucidated.
Research implications
It is not yet known whether amniotic fluid miRNA profiles change throughout gestation or following fetal intervention with SFLP. The specific source of the miRNAs present in amniotic fluid is also not understood, and miRNAs present in amniotic fluid may come from fetal urine, fetal skin cells, fetal respiratory secretions, or from transplacental transfer from the maternal circulation. Thus, it is unclear whether the donor twin or maternal factors contribute in any way to the recipient amniotic fluid miRNA profile. Furthermore, it is not known how the amniotic fluid miRNA profiles of singletons with other forms of congenital heart disease or hydrops would compare to our TTTS population with cardiomyopathy. The immediate fetal and long-term neonatal impact of altered miRNA expression in the setting of recipient cardiomyopathy is also not known.
An area of active research is whether amniotic fluid miRNA profiles are reflected in maternal serum. miRNAs of placental origin have been found to circulate in maternal blood,52–54 making them potential noninvasive biomarkers of pregnancy disease states.21 In addition, Juracek et al55 identified variations in umbilical cord blood miRNA profiles based on various maternal characteristics such as age, BMI, and smoking status. These findings suggest that the maternal and fetal units are not completely isolated with respect to miRNA.
Strengths and limitations
Strengths of this study include a control group of MCDA pregnancies, which eliminates potential confounding by differences in the miRNA expression in singleton and dizygotic twin gestations. In addition, the control group had TTTS to a severe enough extent that in most cases, the patients had to undergo SFLP, enhancing the relevance of the miRNA differences identified in the setting of severe recipient cardiomyopathy.
Additional strengths include a sample size adequate to detect substantial differences in miRNA expression, even after controlling for multiple comparisons. This study compared the miRNA profiles in the amniotic fluid of twins with TTTS on the basis of recipient cardiomyopathy.
A limitation of our study is the requirement for an invasive procedure to obtain amniotic fluid. In this study, all pregnancies were already undergoing therapeutic entry of the amniotic sac. The usefulness of amniotic fluid miRNAs for predictive or prognostic purposes will depend on numerous clinical factors and further research. For example, an amniocentesis is much less invasive than SFLP owing to the small caliber of a needle used in comparison with a fetoscope, and the risks for amniocentesis may be reasonably balanced against the opportunity to avoid an SFLP, if future studies confirm an ability to stratify patients according to risk based on amniotic fluid biomarkers. Further investigations in which amniotic fluid miRNAs are being correlated with maternal serum miRNAs are ongoing to determine if a noninvasive approach can similarly yield useful information.
In addition, because we only stratified the degree of cardiomyopathy according to RV MPI score as opposed to stratification according to all potential markers of cardiomyopathy, our study has limited generalizability to recipient twins with severe cardiomyopathy based on severe ventricular hypertrophy or AV regurgitation alone. However, as a Doppler index, the MPI is reproducible among experienced sonographers and is quantifiable and has been validated as a tool for assessing fetal ventricular dysfunction.16
Conclusion
Amniotic fluid miRNAs are differentially expressed in TTTS pregnancies with severe cardiomyopathy and TTTS control pregnancies with preserved cardiac function. Further investigation into the use of miRNAs as biomarkers for risk stratification of TTTS pregnancies is warranted.
Supplementary Material
AJOG at a Glance.
Why was this study conducted?
There are currently no clinically useful biomarkers of disease severity in twin-twin transfusion syndrome (TTTS) cases.
Key findings
Several microRNAs demonstrate differential expression in the amniotic fluid of pregnancies with TTTS with severe recipient cardiomyopathy when compared with similar twin pregnancies with preserved cardiac function.
What does this add to what it known?
We have characterized amniotic fluid microRNA in TTTS cases and uncovered additional biological insight into the fetal pathophysiology of recipient cardiomyopathy associated with TTTS. These identified microRNAs may serve as biomarkers of disease severity.
Acknowledgments
This study was funded by the National Institutes of Health under award numbers K24HL150630 to C.C.S. and R01HL139968-01 to S.D.M. and C.C.S. and by the Jack Cooper Millisor Chair in Pediatric Heart Disease to S.D.M. The funding sources were not involved in this research.
Footnotes
This study was presented in part at the 41st annual scientific meeting of the Society for Maternal-Fetal Medicine, Las Vegas, NV, January 25–30, 2021.
References
- 1.Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol 2008;199: 514.e1–8. [DOI] [PubMed] [Google Scholar]
- 2.Society for Maternal-Fetal Medicine, Simpson LL. Twin-twin transfusion syndrome. Am J Obstet Gynecol 2013;208:3–18. [DOI] [PubMed] [Google Scholar]
- 3.Behrendt N, Galan HL. Twin-twin transfusion and laser therapy. Curr Opin Obstet Gynecol 2016;28:79–85. [DOI] [PubMed] [Google Scholar]
- 4.Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin-twin transfusion syndrome. J Perinatol 1999;19:550–5. [DOI] [PubMed] [Google Scholar]
- 5.Habli M, Michelfelder E, Cnota J, et al. Prevalence and progression of recipient-twin cardiomyopathy in early-stage twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2012;39: 63–8. [DOI] [PubMed] [Google Scholar]
- 6.Wohlmuth C, Gardiner HM, Diehl W, Hecher K. Fetal cardiovascular hemodynamics in twin-twin transfusion syndrome. Acta Obstet Gynecol Scand 2016;95:664–71. [DOI] [PubMed] [Google Scholar]
- 7.Shah AD, Border WL, Crombleholme TM, Michelfelder EC. Initial fetal cardiovascular profile score predicts recipient twin outcome in twin-twin transfusion syndrome. J Am Soc Echocardiogr 2008;21:1105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrea C, Alkazaleh F, Ryan G, et al. Prenatal cardiovascular manifestations in the twin-to-twin transfusion syndrome recipients and the impact of therapeutic amnioreduction. Am J Obstet Gynecol 2005;192:892–902. [DOI] [PubMed] [Google Scholar]
- 9.Michelfelder E, Tan X, Cnota J, et al. Prevalence, spectrum, and outcome of right ventricular outflow tract abnormalities in twin-twin transfusion syndrome: a large, single-center experience. Congenit Heart Dis 2015;10: 209–18. [DOI] [PubMed] [Google Scholar]
- 10.Villa CR, Habli M, Votava-Smith JK, et al. Assessment of fetal cardiomyopathy in early-stage twin-twin transfusion syndrome: comparison between commonly reported cardiovascular assessment scores. Ultrasound Obstet Gynecol 2014;43:646–51. [DOI] [PubMed] [Google Scholar]
- 11.Fesslova V, Villa L, Nava S, Mosca F, Nicolini U. Fetal and neonatal echocardiographic findings in twin-twin transfusion syndrome. Am J Obstet Gynecol 1998;179:1056–62. [DOI] [PubMed] [Google Scholar]
- 12.Harkness UF, Crombleholme TM. Twin-twin transfusion syndrome: where do we go from here? Semin Perinatol 2005;29:296–304. [DOI] [PubMed] [Google Scholar]
- 13.Rychik J, Tian Z, Bebbington M, et al. The twin-twin transfusion syndrome: spectrum of cardiovascular abnormality and development of a cardiovascular score to assess severity of disease. Am J Obstet Gynecol 2007;197:392. e1–8. [DOI] [PubMed] [Google Scholar]
- 14.Stirnemann JJ, Mougeot M, Proulx F, et al. Profiling fetal cardiac function in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol 2010;35:19–27. [DOI] [PubMed] [Google Scholar]
- 15.Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function–a study in normals and dilated cardiomyopathy. J Cardiol 1999;26: 357–66. [PubMed] [Google Scholar]
- 16.Friedman D, Buyon J, Kim M, Glickstein JS. Fetal cardiac function assessed by Doppler myocardial performance index (Tei Index). Ultrasound Obstet Gynecol 2003;21:33–6. [DOI] [PubMed] [Google Scholar]
- 17.Mackie FL, Hall MJ, Morris RK, Kilby MD. Early prognostic factors of outcomes in monochorionic twin pregnancy: systematic review and meta-analysis. Am J Obstet Gynecol 2018;219:436–46. [DOI] [PubMed] [Google Scholar]
- 18.Van Mieghem T, Doné E, Gucciardo L, et al. Amniotic fluid markers of fetal cardiac dysfunction in twin-to-twin transfusion syndrome. Am J Obstet Gynecol 2010;202:48.e1–7. [DOI] [PubMed] [Google Scholar]
- 19.Habli M, Cnota J, Michelfelder E, et al. The relationship between amniotic fluid levels of brain-type natriuretic peptide and recipient cardiomyopathy in twin-twin transfusion syndrome. Am J Obstet Gynecol 2010;203:404. e1–7. [DOI] [PubMed] [Google Scholar]
- 20.Fasoulakis Z, Theodora M, Tsirkas I, et al. The role of microRNAs identified in the amniotic fluid. Microrna 2020;9:8–16. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Z, Moley KH, Gronowski AM. Diagnostic potential for miRNAs as biomarkers for pregnancy-specific diseases. Clin Biochem 2013;46:953–60. [DOI] [PubMed] [Google Scholar]
- 22.Topkara VK, Mann DL. Role of microRNAs in cardiac remodeling and heart failure. Cardiovasc Drugs Ther 2011;25:171–82. [DOI] [PubMed] [Google Scholar]
- 23.Du J, Li M, Huang Q, et al. The critical role of microRNAs in stress response: therapeutic prospect and limitation. Pharmacol Res 2019;142:294–302. [DOI] [PubMed] [Google Scholar]
- 24.Mendell JT, Olson EN. MicroRNAs in stress signaling and human disease. Cell 2012;148: 1172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyamoto SD, Karimpour-Fard A, Peterson V, et al. Circulating microRNA as a biomarker for recovery in pediatric dilated cardiomyopathy. J Heart Lung Transplant 2015;34:724–33. [DOI] [PubMed] [Google Scholar]
- 26.Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem 2010;56:1733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy S, Zhao M, Hu DQ, et al. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics 2012;44:562–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sucharov CC, Sucharov J, Karimpour-Fard A, Nunley K, Stauffer BL, Miyamoto SD. Micro-RNA expression in hypoplastic left heart syndrome. J Card Fail 2015;21:83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stauffer BL, Russell G, Nunley K, Miyamoto SD, Sucharov CC. MiRNA expression in pediatric failing human heart. J Mol Cell Cardiol 2013;57:43–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun T, Li W, Li T, Ling S. MicroRNA Profiling of amniotic fluid: evidence of synergy of microRNAs in fetal development. PLoS One 2016;11: e0153950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karaca E, Aykut A, Ertürk B, et al. MicroRNA expression profile in the prenatal amniotic fluid samples of pregnant women with down syndrome. Balkan Med J 2018;35:163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie J, Zhou Y, Gao W, Li Z, Xu Z, Zhou L. The relationship between amniotic fluid miRNAs and congenital obstructive nephropathy. Am J Transl Res 2017;9:1754–63. [PMC free article] [PubMed] [Google Scholar]
- 33.Schuchardt EL, Crombleholme TM, Zuk J, et al. The unique micro-RNA signature in amniotic fluid of recipients fetuses with twin-twin transfusion syndrome cardiomyopathy. Circulation 2018;138:A12747. [Google Scholar]
- 34.Shi J, Bei Y, Kong X, et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics 2017;7:664–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Lu Y, Ong’Achwa MJ, et al. Resveratrol inhibits the TGF- β 1-induced proliferation of cardiac fibroblasts and collagen secretion by downregulating miR-17 in rat. BioMed Res Int 2018;2018:8730593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du WW, Li X, Li T, et al. The microRNA miR-17-3p inhibits mouse cardiac fibroblast senescence by targeting Par4. J Cell Sci 2015;128: 293–304. [DOI] [PubMed] [Google Scholar]
- 37.Zhou M, Cai J, Tang Y, Zhao Q. MiR-17-92 cluster is a novel regulatory gene of cardiac ischemic/reperfusion injury. Med Hypotheses 2013;81:108–10. [DOI] [PubMed] [Google Scholar]
- 38.Shi H, Li J, Song Q, et al. Systematic identification and analysis of dysregulated miRNA and transcription factor feed-forward loops in hypertrophic cardiomyopathy. J Cell Mol Med 2019;23:306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu H, Liu Z, Zhou L. Roles of MIR-17-92 cluster in cardiovascular development and common diseases. BioMed Res Int 2017;2017: 9102909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danielson LS, Park DS, Rotllan N, et al. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J 2013;27: 1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang S, Huang MLH, Richardson DR. Treatment of dilated cardiomyopathy in a mouse model of Friedreich’s ataxia using N-acetylcysteine and identification of alterations in micro-RNA expression that could be involved in its pathogenesis. Pharmacol Res 2020;159: 104994. [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Zhang S, Guo C, Li J, Sang W. Downregulation of miR-200c protects cardiomyocytes from hypoxia-induced apoptosis by targeting GATA-4. Int J Mol Med 2017;39: 1589–96. [DOI] [PubMed] [Google Scholar]
- 43.Singh GB, Raut SK, Khanna S, et al. MicroRNA-200c modulates DUSP-1 expression in diabetes-induced cardiac hypertrophy. Mol Cell Biochem 2017;424:1–11. [DOI] [PubMed] [Google Scholar]
- 44.Hu S, Cheng M, Guo X, et al. Down-regulation of miR-200c attenuates AngII-induced cardiac hypertrophy via targeting the MLCK-mediated pathway. J Cell Mol Med 2019;23: 2505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu P, Yang M, Ren H, et al. Long noncoding RNA MALAT1 downregulates cardiac transient outward potassium current by regulating miR-200c/HMGB1 pathway. J Cell Biochem 2018;119:10239–49. [DOI] [PubMed] [Google Scholar]
- 46.Wang K, Long B, Zhou LY, et al. CARL lncRNA inhibits anoxia-induced mitochondrial fission and apoptosis in cardiomyocytes by impairing miR-539-dependent PHB2 down-regulation. Nat Commun 2014;5:3596. [DOI] [PubMed] [Google Scholar]
- 47.Jeanson-Leh L, Lameth J, Krimi S, et al. Serum profiling identifies novel muscle miRNA and cardiomyopathy-related miRNA biomarkers in golden retriever muscular dystrophy dogs and duchenne muscular dystrophy patients. Am J Pathol 2014;184: 2885–98. [DOI] [PubMed] [Google Scholar]
- 48.Muthusamy S, DeMartino AM, Watson LJ, et al. MicroRNA-539 is up-regulated in failing heart, and suppresses O-GlcNAcase expression. J Biol Chem 2014;289:29665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dassanayaka S, Brittian KR, Long BW, et al. Cardiomyocyte Oga haploinsufficiency increases O-GlcNAcylation but hastens ventricular dysfunction following myocardial infarction. PLoS One 2020;15:e0242250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackie FL, Baker BC, Beggs AD, Stodolna A, Morris RK, Kilby MD. MicroRNA changes in maternal serum from pregnancies complicated by twin-twin transfusion syndrome: a discovery study. Prenat Diagn 2019;39: 616–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hui L, Wick HC, Moise KJ, et al. Global gene expression analysis of amniotic fluid cell-free RNA from recipient twins with twin-twin transfusion syndrome. Prenat Diagn 2013;33:873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Whitehead CL, Walker SP, Tong S. Measuring circulating placental RNAs to non-invasively assess the placental transcriptome and to predict pregnancy complications. Prenat Diagn 2016;36:997–1008. [DOI] [PubMed] [Google Scholar]
- 53.Higashijima A, Miura K, Mishima H, et al. Characterization of placenta-specific microRNAs in fetal growth restriction pregnancy. Prenat Diagn 2013;33:214–22. [DOI] [PubMed] [Google Scholar]
- 54.Luo SS, Ishibashi O, Ishikawa G, et al. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol Reprod 2009;81: 717–29. [DOI] [PubMed] [Google Scholar]
- 55.Juracek J, Piler P, Janku P, Radova L, Slaby O. Identification of microRNA signatures in umbilical cord blood associated with maternal characteristics. PeerJ 2019;7:e6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


