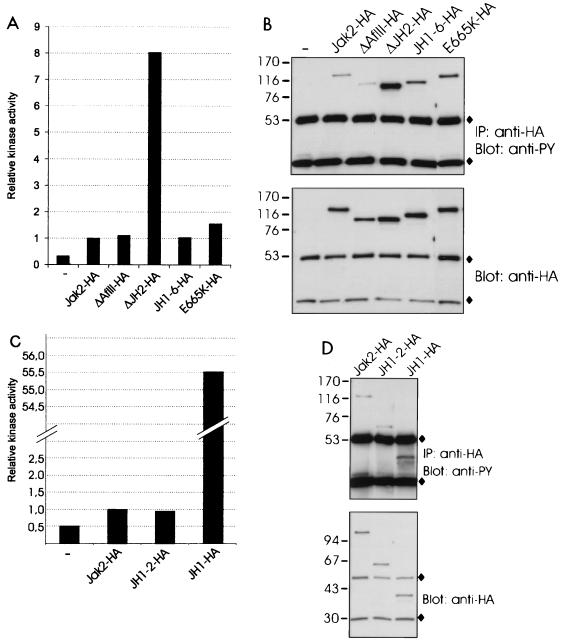
FIG. 2.
Deletion of JH2 increases the catalytic activity of Jak2. (A) 293T cells were transfected with expression plasmids for Jak2-HA, ΔAflII-HA, ΔJH2-HA, JH1-6-HA, and E665K-HA or left untransfected (—). The lysates were immunoprecipitated with anti-HA antibody, and aliquots were subjected to in vitro kinase assays with Stat1-derived peptide and [γ-32P]ATP as substrates. The peptides were separated by SDS–20% PAGE followed by quantification with a PhosphorImager. Relative catalytic activities are shown. Radioactivity incorporated in the Stat1 peptide by the different Jak2 proteins was normalized to the radioactivity incorporated in the Stat1 peptide by Jak2-HA, which was set at 1. (B) Aliquots of the same immunoprecipitates (IP) were analyzed by SDS–4 to 15% PAGE and blotted with antiphosphotyrosine (anti-PY) or anti-HA antibodies. (C) 293T cells were transfected with expression plasmids for Jak2-HA, JH1-2-HA, and JH1-HA or left untransfected. The lysates were immunoprecipitated with anti-HA antibody and analyzed in in vitro kinase assays as in panel A. (D) Aliquots of the same immunoprecipitates were analyzed by SDS–4 to 15% PAGE and blotted with antiphosphotyrosine or anti-HA antibodies. (B and D) ⧫, immunoglobulin chains. The mobilities of the molecular mass markers (in kilodaltons) are shown on the left.