Abstract
Background
Pulmonary exacerbations (PEx) in people with cystic fibrosis (PwCF) are associated with significant morbidity. While standard PEx treatment for PwCF with Pseudomonas aeruginosa infection includes two IV antipseudomonal antibiotics, little evidence exists to recommend this approach. This study aimed to compare clinical outcomes of single versus double antipseudomonal antibiotic use for PEx treatment.
Methods
Retrospective cohort study using the linked CF Foundation Patient Registry-Pediatric Health Information System dataset. PwCF were included if hospitalized between 2007 and 2018 and 6–21 years of age. Regression modeling accounting for repeated measures was used to compare lung function outcomes between single versus double IV antipseudomonal antibiotic regimens using propensity-score weighting to adjust for relevant confounding factors.
Results
Among 10,660 PwCF in the dataset, we analyzed 2,578 PEx from 1,080 PwCF, of which 455 and 2,123 PEx were treated with 1 versus 2 IV antipseudomonal antibiotics, respectively. We identified no significant differences between PEx treated with 1 versus 2 IV antipseudomonal antibiotics either in change between pre- and post-PEx percent predicted forced expiratory volume in one second (ppFEV1) (–0.84%, [95% CI –2.25, 0.56]; P = 0.24), odds of returning to ≥90% of baseline ppFEV1 within 3 months following PEx (Odds Ratio 0.83, [95% CI 0.61, 1.13]; P = 0.24) or time to next PEx requiring IV antibiotics (Hazard Ratio 1.04, [95% CI 0.87, 1.24]; P = 0.69).
Conclusions
Use of 2 IV antipseudomonal antibiotics for PEx treatment in young PwCF was not associated with greater improvements in measured respiratory and clinical outcomes compared to treatment with 1 IV antipseudomonal antibiotic.
Keywords: antibiotics, cystic fibrosis, Pseudomonas aeruginosa, pulmonary exacerbations
In this study, we found that the use of 2 intravenous (IV) antipseudomonal antibiotics for pulmonary exacerbation treatment among people with cystic fibrosis was not associated with improved clinical outcomes compared with use of 1 IV antipseudomonal antibiotic.
Pulmonary exacerbations (PEx) in people with cystic fibrosis (PwCF) are associated with significant morbidity, including decreased quality of life, lung function decline, and weight loss [1-4]. Mild to moderate PEx are often treated with oral antibiotics and aggressive airway clearance, while severe PEx typically require intravenous (IV) antibiotics [5]. In those with Pseudomonas aeruginosa (Pa) infection, simultaneous treatment with 2 IV antipseudomonal antibiotics is common, based on the reasoning that this strategy enhances microbicidal activity and reduces selection of resistant organisms [6-9].
Cystic Fibrosis Foundation PEx guidelines, based on consensus and expert opinion, also note that standard of care is to use 2 IV antipseudomonal antibiotics [5], and a recent study of those hospitalized for PEx treatment found that 2 IV antipseudomonal antibiotics were used in approximately 95% of cases [10]. However, a Cochrane review of 8 clinical trials involving 356 participants could not determine whether multiple IV antipseudomonal antibiotic treatment was associated with greater lung function improvement compared with a single antibiotic regimen [11]. The use of a single antipseudomonal antibiotic has the potential benefit of reducing antibiotic-related toxicities [12, 13], which could limit the lifetime antibiotic exposure of PwCF and the associated risks of drug reactions, uncommon or cumulative toxicity, and possibly resistance.
Using the CF Foundation Patient Registry [14] (CFFPR)–Pediatric Health Information System [15] (PHIS; Children’s Hospital Association, Lenexa, KS) linked dataset described below [16], we aimed to evaluate the PEx treatment of PwCF and chronic Pa infection and to determine if better clinical outcomes are achieved in those treated with double vs single IV antipseudomonal antibiotic strategies. We tested the hypothesis that, among PwCF aged 6–21 years with chronic Pa infection, the use of 2 IV antipseudomonal antibiotics for PEx treatment would be associated with improved clinical outcomes compared with treatment with 1 IV antipseudomonal antibiotic.
METHODS
Study Design
We performed a retrospective cohort study using data from the CFFPR-PHIS linked dataset, which contains clinical and demographic information from 10 660 US children and adolescents with CF from 2005 through 2018 [16]. This linked dataset provides comprehensive outpatient and in-hospital data from PwCF to systematically capture both inpatient antibiotics for PEx treatment and clinical outcomes (ie, lung function) in children with CF. Important demographic (eg, age, sex) and clinical characteristics (eg, lung function, CF microbiology) were obtained from the CFFPR, while in-hospital medication administration data (ie, use of 1 vs 2 IV antipseudomonal antibiotics) and length of stay were captured from PHIS. CFFPR data are collected using standardized forms and manually entered by staff at each care site who review medical records and/or patient-reported forms [14], while PHIS data are electronically updated on a quarterly basis and subjected to continuous data quality reviews [15]. Exact/close match and PHIS-only encounters were included for analysis [17].
Our primary study aims were to determine if the use of 2 IV antipseudomonal antibiotics for PEx treatment would be associated with a greater pre- to post-PEx change in percent predicted forced expiratory volume in 1 second (ppFEV1), a higher odds of returning to ≥90% of baseline ppFEV1, and a longer time to the next PEx requiring IV antibiotics compared with PEx treatment with 1 IV antipseudomonal antibiotic.
Study Definitions
As has been done previously [18], the baseline ppFEV1 was defined as the highest ppFEV1 measurement recorded within 6 months prior to a study PEx. Pre-PEx treatment lung function was defined as the lowest ppFEV1 recorded within 30 days prior to admission up to the first in-hospital ppFEV1 measurement. Post-PEx treatment lung function was defined as the last in-hospital ppFEV1 measurement or the earliest ppFEV1 measurement recorded after the hospital stay up to day 42, whichever was highest. To evaluate return to baseline lung function, the best ppFEV1 within 3 months following the study PEx was compared with the baseline ppFEV1 [19]. Time to next PEx requiring IV antibiotics was defined as the time between the study PEx discharge date and subsequent hospitalization requiring IV antibiotics. In the event no subsequent PEx occurred, PwCF were censored at the last CFFPR encounter date. Chronic Pa infection was defined as Pa culture positivity in at least 2 age quarters per year for 2 consecutive years prior to a study PEx [20]. Antibiotic exposure was defined as use of either 1 or 2 IV antipseudomonal antibiotics on at least 80% of hospital days (IV antipseudomonal antibiotics used are listed in the Supplementary Materials).
Inclusion and Exclusion Criteria
PwCF and chronic Pa infection were included if hospitalized for a PEx between 2007 and 2018, were aged 6–21 years, and received either 1 or 2 IV antipseudomonal antibiotics within 48 hours of admission. To ensure each participant had an opportunity to return to ≥90% of baseline ppFEV1, eligible PEx required a minimum drop in ppFEV1 of ≥3% from baseline to admission. Individuals with a history of solid organ transplant or malignancy were excluded. Exclusion criteria for each study PEx included a culture positive for nontuberculous mycobacteria or Burkholderia cepacia complex species within the preceding 12 months, intensive care unit stay, PEx requiring IV antibiotics within the preceding 3 months, or length of stay <5 or >35 days. We elected to exclude PEx requiring IV antibiotics within the preceding 3 months to allow a participant to recover to lung function baseline prior to another PEx event.
Analytic Methods
Cohort demographic and clinical characteristics were described numerically using medians and interquartile ranges (IQRs) for continuous variables and counts and proportions for categorical variables. The unit of analysis was the study PEx, and PwCF were allowed to have multiple study PEx. To account for correlations between multiple PEx per person, repeated data analysis methods were used. Pre- to post-PEx change in ppFEV1 was regressed on IV antipseudomonal antibiotic exposure (1 vs 2) using a linear mixed-effect model containing random intercept terms for study site and participants. Return to baseline ppFEV1 was regressed on IV antipseudomonal antibiotic exposure (1 vs 2) using a generalized linear model based on generalized estimating equations assuming a binomial distribution and logit link function. The association between number of IV antipseudomonal antibiotics (1 vs 2) and time to next PEx requiring IV antibiotics was evaluated using Kaplan-Meier curves and a conditional Cox proportional hazard regression model with robust standard errors and baseline hazard stratified by current study PEx number within patient (1, 2, 3, or greater). This stratification was performed to ensure that PwCF were included in the correct risk set depending on their prior PEx history. A full list of model covariates is available in the Supplementary Materials. Variability in antipseudomonal antibiotic use (1 vs 2) across PHIS hospitals was accounted for by including site as a random effect and using an exchangeable correlation matrix.
Due to concerns that confounding by indication (ie, PwCF with greater baseline disease comorbidity, for example, renal or hepatic impairment might be more likely to receive 1 IV due to concerns for antibiotic-related toxicity), we used stabilized inverse probability of treatment weighting (as used in prior analyses to address confounding by indication [18, 21]) to reweight our sample and balance measured confounders between groups (model details available in the Supplementary Materials). For PwCF with 2 or more PEx in the dataset, each study PEx could have either 1 or 2 IV antipseudomonal antibiotics. The data were structured at the study PEx level, where each patient had a minimum of 1 study PEx. Mixed effects models were used to account for repeat observations per patient and correlations between these observations.
Kaplan-Meier curves and Schoenfeld residual plots were used to assess the proportional hazards assumption for the Cox model assessing time to next PEx. There was no evidence of violation of the proportional hazards assumption (see Supplementary Figures 1–4). For change in pre- to post-PEx ppFEV1, we examined scatterplots to assess the linearity assumption. For both change in pre- to post-PEx ppFEV1 and return to ≥90% of baseline ppFEV1, histograms were used to assess the normality of model residuals.
The Seattle Children’s Hospital Institutional Review Board approved the study.
Additional Analyses
Due to concerns related to misclassification of antibiotic exposure, we performed a subset analysis that defined antibiotic exposure as use of either 1 or 2 IV antipseudomonal antibiotics in 100% (rather than only 80%) of hospital days. A separate sensitivity analysis compared clinical outcomes among PEx treated with 1 vs 2 IV antipseudomonal antibiotics with or without the addition of an inhaled antibiotic. We performed an additional sensitivity analysis that excluded PEx treated with oral quinolones due to the fact that oral and IV quinolones have similar bioavailability. Last, a separate analysis using the antibiotic spectrum score [22] compared regimens that contained antibiotics categorized as broader (meropenem/tobramycin and piperacillin-tazobactam/tobramycin) vs narrower (ceftazidime/tobramycin and cefepime/tobramycin) spectrum among a subset of PEx treated with 2 IV antipseudomonal antibiotics.
RESULTS
Primary Analysis
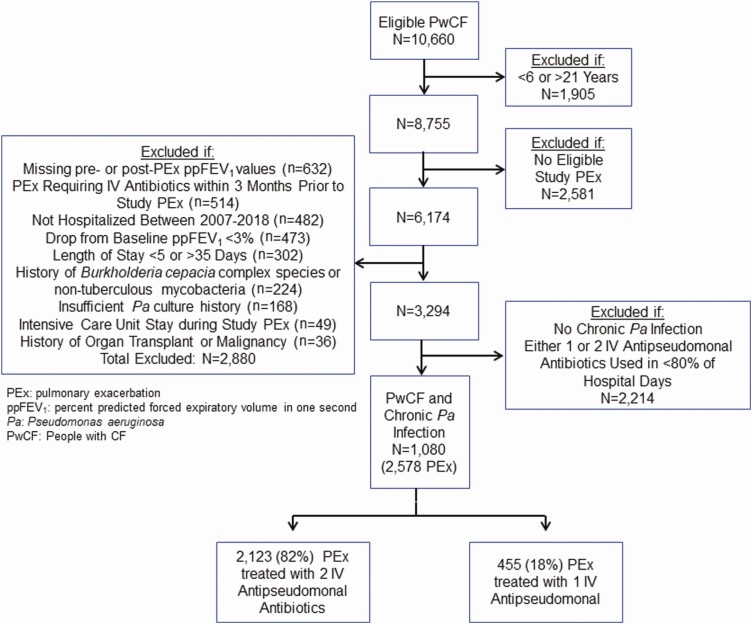
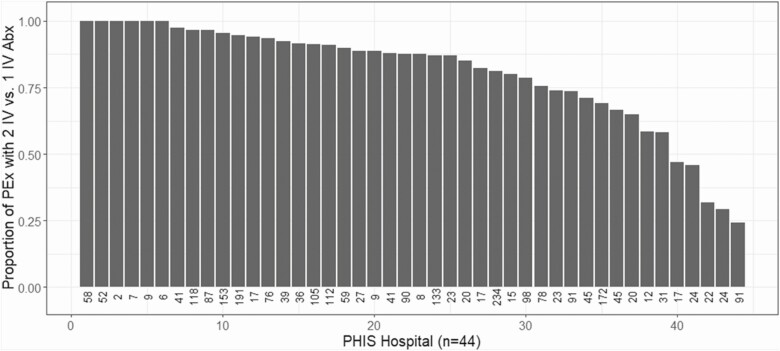
After applying inclusion and exclusion criteria, 1080 PwCF and chronic Pa infection contributed 2578 study PEx between 2007 and 2018 (Figure 1) and 591 (55%) participants contributed multiple PEx events. Among these, 2123 PEx treated with 2 IV antipseudomonal antibiotics and 455 PEx treated with 1 IV antipseudomonal antibiotic were available for analysis. Median length of stay was the same (11 days; IQR, 8–14) among PEx treated with either 1 or 2 IV antipseudomonal antibiotics. When only 1 IV antipseudomonal antibiotic was administered, it was most commonly a beta-lactam (Supplementary Table 1). Among all PHIS hospitals, there was substantial variability in the use of 1 vs 2 IV antipseudomonal antibiotics for CF PEx treatment (Figure 2). PwCF and chronic Pa infection treated with 1 IV antipseudomonal antibiotic had a lower baseline ppFEV1 (74.9%; IQR, 60.0–87.8) and were more likely treated for at least 2 PEx in the 12 months prior to a study PEx (54%) compared with PwCF treated with 2 IV antipseudomonal antibiotics (ppFEV1 78.8%; IQR, 62.8–91.5) and 40% treated for at least 2 PEx in the 12 months prior to a study PEx (Table 1). In addition, PwCF treated with 1 IV antipseudomonal antibiotic had a higher prevalence of methicillin-resistant Staphylococcus aureus (MRSA) and multidrug-resistant Pa (MDR-Pa) infection (45% and 16%, respectively) compared with PwCF treated with 2 IV antipseudomonal antibiotics (35% and 8%, respectively). Oral antipseudomonal antibiotics were used in 14% of PEx treated with 1 IV antipseudomonal antibiotic compared with 2% of PEx treated with 2 IVs (a time-varying covariate in the inverse probability of treatment weighting models).
Figure 1.
Flow diagram illustrating cohort selection. Abbreviations: IV, intravenous; Pa, Pseudomonas aeruginosa; PEx, pulmonary exacerbation; ppFEV1, percent predicted forced expiratory volume in 1 second; PwCF, people with cystic fibrosis.
Figure 2.
Proportion of PEx treated with 2 vs 1 IV antipseudomonal antibiotics by PHIS hospital. The number below each PHIS hospital indicates the number of PEx included in the analysis from each PHIS hospital. Abbreviations: Abx, antibiotics; IV, intravenous; PEx, pulmonary exacerbation; PHIS, Pediatric Health Information System.
Table 1.
Pulmonary Exacerbation–Level Characteristics of the Cohort by Antibiotic Exposure
| Variable | 2 IV Antipseudomonal Antibiotics (N = 2123) | 1 IV Antipseudomonal Antibiotic (N = 455) |
|---|---|---|
| Demographic variables | ||
| Age (years) | 16.1 (13.3–18.0) | 16.2 (13.3–18.1) |
| Sex (male) | 936 (44%) | 210 (46%) |
| Ethnicity | ||
| Non-Hispanic White | 1602 (75%) | 286 (63%) |
| Hispanic White | 310 (15%) | 128 (28%) |
| Other/Unknown | 211 (10%) | 41 (9%) |
| One class I–III mutation | 87 (4%) | 8 (2%) |
| Two class I–III mutations | 1774 (84%) | 362 (80%) |
| Other/Unknown genotype | 258 (12%) | 85 (19%) |
| Public insurance | 1337 (63%) | 308 (68%) |
| Private insurance | 759 (36%) | 138 (30%) |
| Other insurance | 27 (1%) | 9 (2%) |
| Clinical variables | ||
| Baselinea ppFEV1 | 78.8% (62.8–91.5) | 74.9% (60.0–87.8) |
| Admissionb ppFEV1 | 60.5% (46.0–74.8) | 58.4% (44.3–70.3) |
| Body mass index percentile | 31.2% (10.7–57.5) | 37.1% (15.2–62.2) |
| Cystic fibrosis–related diabetes | 715 (34%) | 157 (35%) |
| Pa at most recent culture | 1633 (77%) | 357 (78%) |
| Multidrug-resistant Pa infection | 177 (8%) | 72 (16%) |
| Methicillin-sensitive Staphylococcus aureus infection | 478 (23%) | 97 (21%) |
| Methicillin-resistant S. aureus infection | 753 (35%) | 206 (45%) |
| Stenotrophomonas maltophilia infection | 136 (6%) | 37 (8%) |
| Achromobacter xylosoxidans infection | 69 (3%) | 20 (4%) |
| Number of PEx events | ||
| Requiring IV antibiotics in prior 12 months | ||
| 1 | 722 (34%) | 127 (28%) |
| ≥2 | 853 (40%) | 254 (54%) |
| Medication | ||
| Pancreatic enzymes | 2024 (95%) | 446 (98%) |
| Chronic inhaled antibiotic | 2029 (96%) | 449 (99%) |
| Use in prior 12 months | ||
| Inhaled antibiotic (during study PEx) | 433 (20%) | 248 (55%) |
| Oral antipseudomonal antibiotic (during study PEx) | 49 (2%) | 65 (14%) |
| Non-antipseudomonal oral antibiotic (during study PEx) | 488 (23%) | 82 (18%) |
| Non-antipseudomonal IV antibiotic (during study PEx) | 512 (24%) | 168 (37%) |
| Azithromycin (during study PEx) | 1283 (60%) | 267 (59%) |
| Systemic corticosteroid (during study PEx) | 281 (13%) | 87 (19%) |
| Cystic fibrosis transmembrane regulator modulator (during study PEx) | 233 (11%) | 49 (11%) |
Abbreviations: IV, intravenous; Pa, Pseudomonas aeruginosa; PEx, pulmonary exacerbation; ppFEV1, percent predicted forced expiratory volume in 1 second.
aDefined as the highest ppFEV1 recorded within 6 months prior to a study PEx.
bDefined as the lowest ppFEV1 recorded within 30 days prior to admission up to the first in-hospital ppFEV1 measurement.
The median drop in ppFEV1 on admission from baseline was 13.8% (IQR, 8.6–20.6) and 14.5% (IQR, 9.1–21.7) among PEx treated with 1 vs 2 IV antipseudomonal antibiotics, respectively, while median pre- to post-PEx change in ppFEV1 was 12.2% (IQR, 5.5–22.9) and 13.9% (IQR, 6.5–25.5) among PEx treated with 1 vs 2 IV antipseudomonal antibiotics, respectively. An identical proportion (79%) of PEx treated with either 1 or 2 IV antipseudomonal antibiotics had return to ≥90% of baseline ppFEV1. Among those in this cohort, 88% had a subsequent PEx; 12% were censored at the last CFFPR encounter date (median time to censor was 1110 days (IQR, 234–2292). The median time to next PEx requiring IV antibiotics was similar for each treatment group (117 days; IQR, 69–219) for PEx treated with 1 IV antipseudomonal antibiotic and 142 days (IQR, 80–259) for PEx treated with 2 IV antipseudomonal antibiotics.
Table 2 presents the inverse probability of treatment weighting adjusted results from the linear mixed-effects (pre- to post-PEx treatment change in ppFEV1), generalized estimating equation logistic regression (return to ≥90% of baseline ppFEV1), and Cox proportional hazards regression (time to next PEx requiring IV antibiotics) models. PEx treated with 1 IV antipseudomonal antibiotic had no statistically significant differences in pre- to post-PEx treatment ppFEV1 (–0.84%; 95% confidence interval [CI], –2.25 to .56; P = .24), odds of returning to ≥90% of baseline ppFEV1 (odds ratio [OR], 0.83; 95% CI, .61 to 1.13; P = .24), or time to next PEx requiring IV antibiotics (hazard ratio [HR], 1.04; 95% CI, .87 to 1.24; P = .69) compared with treatment with 2 IV antipseudomonal antibiotics.
Table 2.
Comparison of Pre- to Post-Pulmonary Exacerbation (PEx) Treatment Change in Percent Predicted Forced Expiratory Volume in 1 Second (ppFEV1), Return to ≥90% of Baseline ppFEV1, and Time to Next PEx Requiring Intravenous (IV) Antibiotics for PEx Treated With 1 vs 2 IV Antipseudomonal Antibiotics
| Variable | IPTW Adjusteda | P value |
|---|---|---|
| Change in ppFEV 1 | ||
| Estimate (95% CI) b | ||
| 1 IV Antipseudomonal Antibiotic (reference: 2 IV) | −0.84% (−2.25, 0.56) | 0.24 |
| Return to ≥90% of Baseline ppFEV 1 | ||
| Odds Ratio (95% CI) c | ||
| 1 IV Antipseudomonal Antibiotic (reference: 2 IV) | 0.83 (0.61, 1.13) | 0.24 |
| Time to Next PEx | ||
| Hazard Ratio (95% CI) d | ||
| 1 IV Antipseudomonal Antibiotic (reference: 2 IV) | 1.04 (0.87, 1.24) | 0.69 |
Abbreviations: CI, confidence interval; IV, intravenous; IPTW (Inverse probability of treatment weighting); ppFEV1 (percent predicted forced expiratory volume in one second).
aThese models contain length of stay, non-antipseudomonal antibiotic switching, and oral antipseudomonal antibiotic switching as covariates.
bLinear mixed-effect model.
cGeneralized estimating equation logistic regression model.
dCox-proportional hazard regression model.
Additional Analyses
When PEx were restricted to those with 100% of hospital days receiving either 1 or 2 IV antipseudomonal antibiotics (ie, no change in antibiotic exposure during the hospitalization), 367 PEx treated with 1 IV antipseudomonal antibiotic and 1522 PEx treated with 2 IV antipseudomonal antibiotics were available for analysis. No statistically significant differences were seen in pre- to post-PEx treatment ppFEV1 (–0.57%; 95% CI, –2.88 to 1.74; P = .63), odds of returning to ≥90% of baseline ppFEV1 (OR, 0.79; 95% CI, .56 to 1.11; P = .17), or time to next PEx requiring IV antibiotics (HR, 0.99; 95% CI, .81 to 1.22; P = .96) among PEx treated with 1 vs 2 IV antipseudomonal antibiotics.
We previously studied the effects of adding inhaled antibiotics to IV antibiotic therapy for PEx treatment and found no clinical benefits [18]. To determine whether inhaled antibiotics had differential effects among our 2 treatment groups, we performed a sensitivity analysis that reclassified antibiotic exposure as 1 vs 2 IV antipseudomonal antibiotics with or without the addition of an inhaled antibiotic. Supplementary Tables 2–4 illustrate results of the inverse probability of treatment weighting regression models for this sensitivity analysis. Similar to the primary analysis, no statistically significant differences were seen in the pre- to post-PEx treatment ppFEV1 or in the odds of recovering to ≥90% of baseline ppFEV1 among the 4 antibiotic exposure groups. When compared with the reference group (2 IV antipseudomonal antibiotics without an inhaled antibiotic), the only antibiotic exposure group with an increased hazard of future PEx was treatment with 1 IV and 1 inhaled antipseudomonal antibiotic (HR, 1.31; 95% CI, 1.06 to 1.63; P = .014). Similar to our primary analysis, our sensitivity analysis in which PEx treated with oral quinolone antibiotics were removed did not find any statistically significant differences in any of the 3 clinical outcomes (Supplementary Table 5).
Finally, we performed a separate analysis to compare outcomes of broader- vs narrower-spectrum antipseudomonal antibiotic regimens among a subset of PEx treated with 2 IV antipseudomonal antibiotics. Supplementary Table 6 illustrates the PEx-level characteristics among the 4 IV antibiotic pairs [22]. In inverse probability of treatment weighting regression models, there were no statistically significant differences in pre- to post-PEx treatment ppFEV1 (0.80%; 95% CI, –.56 to 2.16; P = .90) or odds of returning to ≥90% of baseline ppFEV1 (OR, 0.99; 95% CI, .74 to 1.32; P = .93) between PEx treated with narrower- vs broader-spectrum IV antipseudomonal antibiotics. PEx treated with narrower-spectrum IV antipseudomonal antibiotics had a reduced hazard for future PEx requiring IV antibiotics (HR, 0.86; 95% CI, .76 to 0.98; P = .021) when compared with PEx treated with broader-spectrum IV antipseudomonal antibiotics (Table 3).
Table 3.
Comparison of Pre- to Post-Pulmonary Exacerbation (PEx) Treatment Change in ppFEV1, Return to ≥90% of Baseline ppFEV1, and Time to Next PEx Requiring Intravenous (IV) Antibiotics between PEx Treated with Narrower-Spectrum versus Broader-Spectrum IV Antipseudomonal Antibiotics
| Variable | Inverse Probability of Treatment Weighting–Adjusteda | P value |
|---|---|---|
| Change in ppFEV 1 | ||
| Estimate (95% CI) b | ||
| Broader-spectrum (reference: Narrower) | 0.80% (−0.56, 2.16) | 0.25 |
| Return to ≥90% of Baseline ppFEV 1 | ||
| Odds Ratio (95% CI) c | ||
| Broader-spectrum (reference: Narrower) | 0.99 (0.74, 1.32) | 0.93 |
| Time to Next PEx | ||
| Hazard Ratio (95% CI) d | ||
| Broader-spectrum (reference: Narrower) | 0.86 (0.76, 0.98) | 0.02 |
Narrow-spectrum includes ceftazidime/tobramycin and cefepime/tobramycin. Broaderspectrum includes meropenem/tobramycin and piperacillin-tazobactam/tobramycin.
Abbreviations: CI, confidence interval; ppFEV1, percent predicted forced expiratory volume in 1 second.
aThese models contain length of stay, non-antipseudomonal antibiotic switching, and oral antipseudomonal antibiotic switching as covariates.
bLinear mixed-effect model.
cGeneralized estimating equation logistic regression model.
dCox-proportional hazard regression model.
DISCUSSION
In contrast to our hypothesis, this retrospective observational study of more than 2500 PEx from more than 1000 PwCF aged <21 years with chronic Pa infection found no statistically significant differences in well-established clinical outcomes following PEx treatment with 1 vs 2 IV antipseudomonal antibiotics. PwCF who were treated with 1 IV antipseudomonal antibiotic tended to be sicker (eg, lower baseline ppFEV1, more prior PEx requiring IV antibiotics, higher infection rate with MRSA and MDR-Pa) upon PEx treatment, consistent with confounding by indication. One possible explanation for these observed differences is that clinicians prescribed 1 IV antipseudomonal antibiotic to limit additional IV antibiotic exposure among PwCF with a history of antibiotic-related adverse events (eg, acute hepatic impairment, acute kidney injury). We used inverse probability of treatment weighting in an attempt to rigorously address this bias. This method allows for the balance of known covariates during study design and prior to performing the analyses [23, 24].
Current CF Foundation PEx guidelines note that not enough evidence exists to conclude whether a single IV antipseudomonal antibiotic strategy is therapeutically equivalent to combination antibiotics for PEx treatment [5]. Potential advantages to double IV therapy include reducing the selection of antibiotic-resistant organisms, synergistic antimicrobial effects, and an ability to target non-Pa gram-negative organisms [6-9]. However, there is evidence that combination antibiotic therapy actually predisposes to antimicrobial resistance. For example, in vitro data comparing the development of Pa resistance during combination therapy with ceftazidime and ciprofloxacin compared with single-drug therapy noted more frequent selection by double-antibiotic treatment of broad-spectrum resistance to both antibiotics in the combination and to unrelated antibiotics from different drug classes [25]. The development of multidrug-resistant Pa has important clinical implications, as it has been associated with CF-related lung disease progression [26].
Only 1 randomized trial has compared 1 vs 2 antipseudomonal antibiotics for PEx treatment of PwCF with Pa infection [27]. This study from 1999 compared PEx treatment with azlocillin/tobramycin to azlocillin/placebo in 76 PwCF. These adults with CF had severe obstructive lung disease (mean admission ppFEV1 was 34%–38%), in contrast to our pediatric cohort for whom the median baseline ppFEV1 was 74%–79%. In the prior randomized trial, no significant differences were found in change in ppFEV1 or exacerbation score, although time to PEx readmission was longer in the group that received azlocillin/tobramycin. In addition, consistent with the in vitro results discussed above, more tobramycin-resistant Pa isolates were seen among PwCF treated with combination therapy. In comparison, in our study, we found no increased hazard of future PEx among PwCF treated with 1 IV antipseudomonal antibiotic compared with treatment with 2 IV antipseudomonal antibiotics. Unfortunately, our study design did not allow us to determine if treatment-emergent antimicrobial resistance was different between the 1 vs 2 IV antipseudomonal antibiotic groups, an important limitation.
In our sensitivity analysis comparing broader- vs narrower-spectrum IV antipseudomonal antibiotic therapy, no statistically significant differences were seen in pre-to post-PEx treatment ppFEV1 or odds of returning to ≥90% of baseline ppFEV1; a reduced hazard for future PEx was seen in the narrower-spectrum antibiotic group compared with broader-spectrum therapy. To our knowledge, only 2 prospective studies (both randomized trials) have compared narrower- vs broader-spectrum IV antibiotic approaches for CF PEx treatment. The first trial (N = 102) evaluated outcomes of PEx treatment with either meropenem/tobramycin or ceftazidime/tobramycin [28]. Significant clinical improvement (change in ppFEV1) occurred in both treatment groups, although a larger proportion of participants in the meropenem/tobramycin group demonstrated ≥15% ppFEV1 improvement by treatment day 7. The second randomized trial (N = 118) also compared meropenem/tobramycin with ceftazidime/tobramycin for PEx treatment [29]. In contrast to the first study, similar improvements in lung function were seen between both groups, although a statistically significant higher elevation in alkaline phosphatase was seen among PwCF treated with meropenem/tobramycin. While broader-spectrum antibiotics generally have more activity against anaerobic bacteria than narrower-spectrum agents, the role anaerobic bacteria play in the development of CF airway disease or PEx development is unclear [30, 31]. Furthermore, broad-spectrum antibiotics may negatively impact health outcomes as they are associated with deleterious alterations to the gut microbiome [32], pediatric obesity [33], and the long-term development of antibiotic resistance [34]. These observations underscore the need for more prospective studies to compare broader- vs narrower-spectrum antibiotic regimens for PEx treatment.
Strengths of this study include the large number of PEx available for analysis and the use of the CFFPR-PHIS dataset that is generalizable to US pediatric PwCF. In addition, results from our primary and sensitivity analyses evaluating clinical outcomes between PEx treated with 1 vs 2 IV antipseudomonal antibiotics all produced similar results, strengthening the overall validity of the study findings.
Importantly, this study has several limitations. The most important relates to confounding by indication. While we attempted to minimize this bias by using inverse probability of treatment weighting, a randomized, controlled trial would be ideal to account for unmeasured confounders. All registry-based studies are prone to missingness and misclassification. While we tried to address this with a sensitivity analysis, misclassification of antibiotic exposure (eg misclassification of an IV antipseudomonal as oral) might have affected study results. We excluded adults with CF since they are less likely to be followed at a PHIS-participating pediatric hospital. Finally, due to CFFPR-PHIS dataset limitations, we were unable to accurately identify important antibiotic-related adverse events, including the incidence of acute kidney injury, allergic reactions, or Clostridium difficile infection, that might have differed between the IV antipseudomonal antibiotic groups.
In conclusion, in this study, we found that a double vs single IV antipseudomonal antibiotic strategy for PEx treatment among PwCF and chronic Pa infection was not associated with improvement in any of the measured respiratory or clinical outcomes. These results illustrate the possibility of limiting IV antibiotic exposure for PEx treatment without sacrificing efficacy (particularly in the current era of highly effective cystic fibrosis transmembrane regulator modulator therapy that is expected to extend life expectancy [35]). This study provides equipoise and a compelling rationale for a prospective randomized, controlled trial to compare efficacy and safety of 1 vs 2 IV antipseudomonal antibiotics in children with CF and chronic Pa infection.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the Cystic Fibrosis Foundation (CFF) for use of CFF Patient Registry data to conduct the study. Additionally, they thank the patients, care providers, and clinic coordinators at cystic fibrosis centers throughout the United States for their contributions to the CFF Patient Registry. In addition, they thank the Children’s Hospital Association team for providing Pediatric Health Information System data for analysis.
Financial support. This work was supported by the CFF (COGEN18Y7 and COGEN 19A0 to J. D. C., COGEN19A0 to A. V. F., COGEN18Y7 to F. O., SINGH19R0 to L. R. H. and R. L. G., and NICHOL18Y3 to D. P. N.) and the National Institutes of Health (NIH) (K24HL141669 to L. R. H. and P30 DK 089507 and 5RO1HL124053 to D. P. N.).
Potential conflicts of interest. R. L. G. reports contracts to cover academic Full-time equivalent (FTE) to conduct clinical trials from Vertex Pharmaceuticals; grants for infrastructure and research support as principal investigator and co-investigator from the CFF; a grant for support of research infrastructure as a co-investigator from the NIH; honoraria for review of clinical research grants from the CFF; and is supported for the cost of travel to attend meetings to review clinical research grants from the CFF all outside the submitted work. D. P. N. reports grants/contracts from the CFF and the NIH, National Heart, Lung, and Blood Institute all outside the submitted work. L. R. H. reports support from the NIH, paid to their institution, and funding paid to their institution, including support for a national resource center they direct on behalf of the CFF that provides laboratory services and study design advice for private and academic researchers interested in developing new treatments for CF infection, from CFF, all during the conduct of the study. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ferkol T, Rosenfeld M, Milla CE. Cystic fibrosis pulmonary exacerbations. J Pediatr 2006; 148:259– 64. [DOI] [PubMed] [Google Scholar]
- 2. Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax 2007; 62:360– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Waters V, Stanojevic S, Atenafu EG, et al. . Effect of pulmonary exacerbations on long-term lung function decline in cystic fibrosis. Eur Respir J 2012; 40:61– 6. [DOI] [PubMed] [Google Scholar]
- 4. Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 2002; 121:64– 72. [DOI] [PubMed] [Google Scholar]
- 5. Flume PA, Mogayzel PJ, Robinson KA, et al. . Cystic fibrosis pulmonary guidelines. Treatment of pulmonary exacerbations. Am J Respir Crit Care Med 2009; 180:802– 8. [DOI] [PubMed] [Google Scholar]
- 6. Watkins J, Francis J, Kuzemko JA. Does monotherapy of pulmonary infections in cystic fibrosis lead to early development of resistant strains of Pseudomonas aeruginosa? Scand J Gastroenterol Suppl 1988; 143:81– 5. [DOI] [PubMed] [Google Scholar]
- 7. Weiss K, Lapointe JR. Routine susceptibility testing of four antibiotic combinations for improvement of laboratory guide to therapy of cystic fibrosis infections caused by Pseudomonas aeruginosa. Antimicrob Agents Chemother 1995; 39:2411– 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saiman L, Mehar F, Niu WW, et al. . Antibiotic susceptibility of multiply resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis, including candidates for transplantation. Clin Infect Dis 1996; 23:532– 7. [DOI] [PubMed] [Google Scholar]
- 9. American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005; 171:388– 416. [DOI] [PubMed] [Google Scholar]
- 10. Cogen JD, Oron AP, Gibson RL, et al. . Characterization of inpatient cystic fibrosis pulmonary exacerbations. Pediatrics 2017; 139:e20162642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elphick HE, Scott A. Single vs combination intravenous anti-pseudomonal antibiotic therapy for people with cystic fibrosis. Cochrane Database Syst Rev 2016:CD002007. doi: 10.1002/14551858.CD002007.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Al-Aloul M, Miller H, Alapati S, Stockton PA, Ledson MJ, Walshaw MJ. Renal impairment in cystic fibrosis patients due to repeated intravenous aminoglycoside use. Pediatr Pulmonol 2005; 39:15– 20. [DOI] [PubMed] [Google Scholar]
- 13. Garinis AC, Cross CP, Srikanth P, et al. . The cumulative effects of intravenous antibiotic treatments on hearing in patients with cystic fibrosis. J Cyst Fibros 2017; 16:401– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knapp EA, Fink AK, Goss CH, et al. . The Cystic Fibrosis Foundation patient registry: design and methods of a national observational disease registry. Ann Am Thorac Soc 2016; 13:1173– 9. [DOI] [PubMed] [Google Scholar]
- 15. Data source: pediatric health information systems database. Lenexa, KS: Children’s Hospital Association. Available at: https://www.childrenshospitals.org/Programs-and-Services/Data-Analytics-and-Research/Pediatric-Analytic-Solutions/Pediatric-Health-Information-System. Accessed 17 May 2021. [Google Scholar]
- 16. Cogen JD, Hall M, Loeffler DR, et al. . Linkage of the Cystic Fibrosis Foundation patient registry with the pediatric health information system database. Pediatr Pulmonol 2019; 54:721– 8. [DOI] [PubMed] [Google Scholar]
- 17. Cogen JD, Faino AV, Onchiri F, Hall M, Fink AK. Evaluation of hospitalization data for the CFFPR-PHIS linked data set. Pediatr Pulmonol 2020; 55:30– 2. [DOI] [PubMed] [Google Scholar]
- 18. Cogen JD, Faino AV, Onchiri F, et al. . Association of inhaled antibiotics in addition to standard IV therapy and outcomes of pediatric inpatient pulmonary exacerbations. Ann Am Thorac Soc 2020; 17:1590– 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sanders DB, Bittner RC, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010; 182:627– 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heltshe SL, Khan U, Beckett V, et al. . Longitudinal development of initial, chronic and mucoid Pseudomonas aeruginosa infection in young children with cystic fibrosis. J Cyst Fibros 2018; 17:341– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cogen JD, Faino AV, Onchiri F, et al. . Effect of concomitant azithromycin and tobramycin use on cystic fibrosis pulmonary exacerbation treatment. Ann Am Thorac Soc 2021; 18:266– 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerber JS, Hersh AL, Kronman MP, Newland JG, Ross RK, Metjian TA. Development and application of an antibiotic spectrum index for benchmarking antibiotic selection patterns across hospitals. Infect Control Hosp Epidemiol 2017; 38:993– 7. [DOI] [PubMed] [Google Scholar]
- 23. Desai RJ, Franklin JM. Alternative approaches for confounding adjustment in observational studies using weighting based on the propensity score: a primer for practitioners. BMJ 2019; 367:l5657. [DOI] [PubMed] [Google Scholar]
- 24. Kahlert J, Gribsholt SB, Gammelager H, Dekkers OM, Luta G. Control of confounding in the analysis phase— an overview for clinicians. Clin Epidemiol 2017; 9:195– 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vestergaard M, Paulander W, Marvig RL, et al. . Antibiotic combination therapy can select for broad-spectrum multidrug resistance in Pseudomonas aeruginosa. Int J Antimicrob Agents 2016; 47:48– 55. [DOI] [PubMed] [Google Scholar]
- 26. Lechtzin N, John M, Irizarry R, Merlo C, Diette GB, Boyle MP. Outcomes of adults with cystic fibrosis infected with antibiotic-resistant Pseudomonas aeruginosa. Respiration 2006; 73:27– 33. [DOI] [PubMed] [Google Scholar]
- 27. Smith AL, Doershuk C, Goldmann D, et al. . Comparison of a beta-lactam alone vs beta-lactam and an aminoglycoside for pulmonary exacerbation in cystic fibrosis. J Pediatr 1999; 134:413– 21. [DOI] [PubMed] [Google Scholar]
- 28. Blumer JL, Saiman L, Konstan MW, Melnick D. The efficacy and safety of meropenem and tobramycin vs ceftazidime and tobramycin in the treatment of acute pulmonary exacerbations in patients with cystic fibrosis. Chest 2005; 128:2336– 46. [DOI] [PubMed] [Google Scholar]
- 29. Latzin P, Fehling M, Bauernfeind A, Reinhardt D, Kappler M, Griese M. Efficacy and safety of intravenous meropenem and tobramycin vs ceftazidime and tobramycin in cystic fibrosis. J Cyst Fibros 2008; 7:142– 6. [DOI] [PubMed] [Google Scholar]
- 30. Muhlebach MS, Hatch JE, Einarsson GG, et al. . Anaerobic bacteria cultured from CF airways correlated to milder disease— a multisite study. Eur Respir J 2018; 52:1800242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sherrard LJ, Bell SC, Tunney MM. The role of anaerobic bacteria in the cystic fibrosis airway. Curr Opin Pulm Med 2016; 22:637– 43. [DOI] [PubMed] [Google Scholar]
- 32. Haak BW, Lankelma JM, Hugenholtz F, Belzer C, de Vos WM, Wiersinga WJ. Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J Antimicrob Chemother 2019; 74:782– 6. [DOI] [PubMed] [Google Scholar]
- 33. Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr 2014; 168:1063– 9. [DOI] [PubMed] [Google Scholar]
- 34. Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, CDC, 2019. [Google Scholar]
- 35. PROMISE study examines trikafta use among CF patients. Available at: https://cysticfibrosisnewstoday.com/2020/06/10/promise-study-trikafta-cystic-fibrosis-. patients. Accessed 30 November 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.