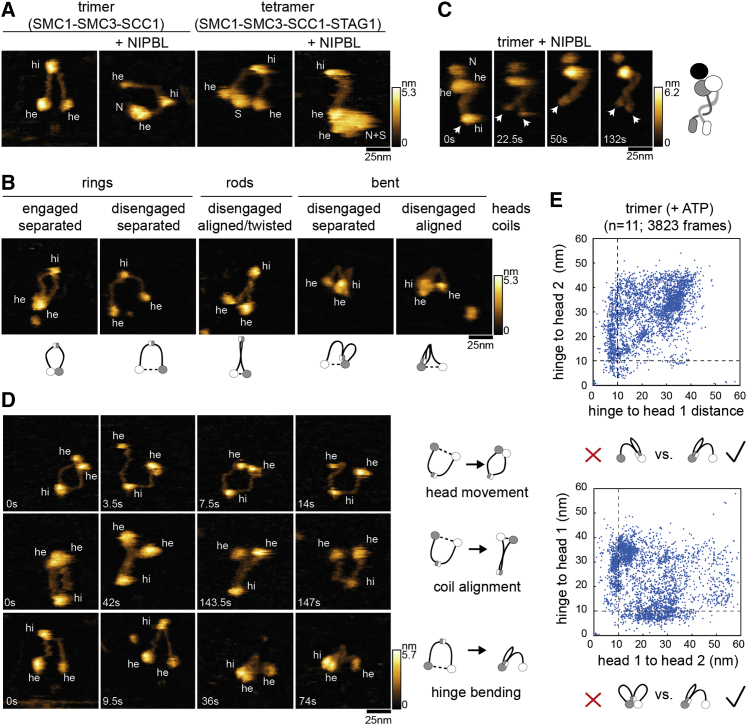
Figure 2.
Conformational changes of cohesin measured by hsAFM
(A) High speed atomic force microscopy recordings (HS-AFM) of isolated trimers, trimers bound to NIPBL, cohesin tetramers, or tetramers bound to NIPBL. Densities corresponding to the hinge (hi), heads (he), NIPBL (N), and STAG1 (S) are indicated.
(B) Major conformations observed in trimeric cohesin in the presence of ATP. The conformations are illustrated by cartoons.
(C) Cohesin trimer bound to NIPBL with twisted coiled coils. Closed and open hinge domains are indicated with arrows.
(D) Trimeric cohesin undergoing head engagements (top), coiled coil alignment (middle), and hinge bending (bottom).
(E) Distribution of head-head and head-hinge distances in trimeric cohesin. n, number of molecules analyzed.