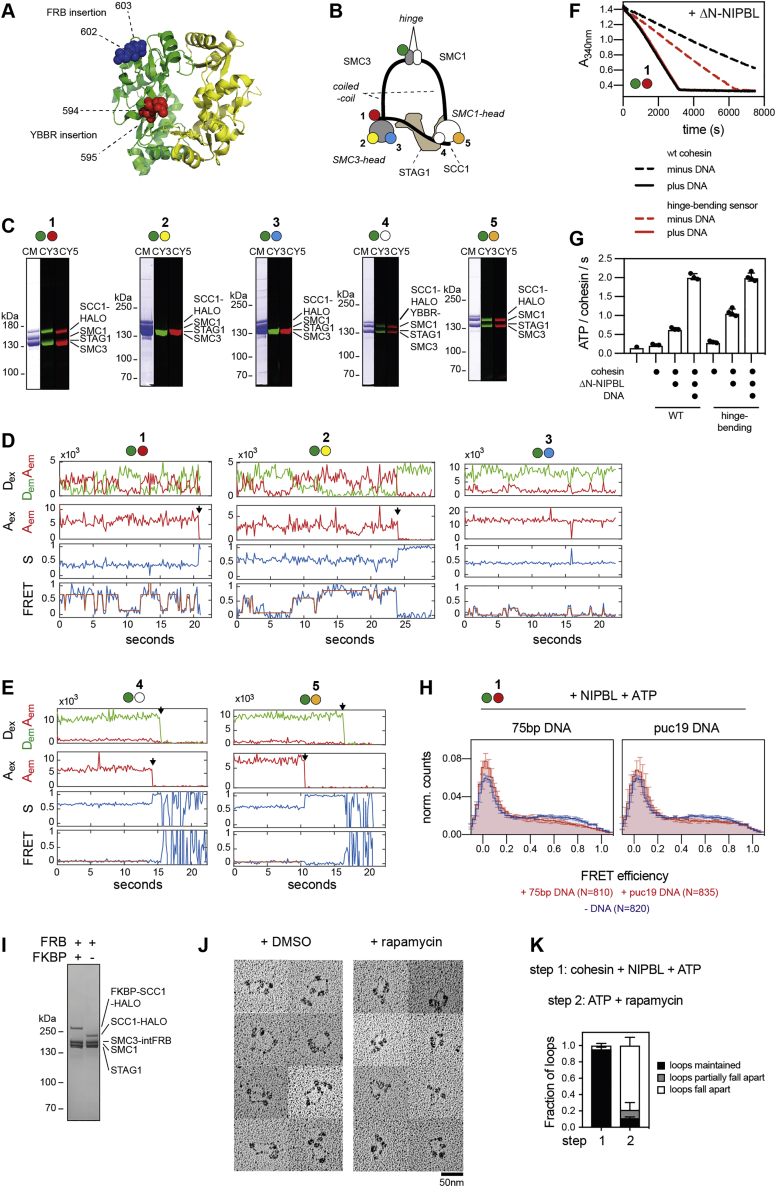
Figure S5.
Kinetics of hinge bending visualized by smFRET, related to Figure 5
A.) Positions of YBBR-tag and FRB-insertions in the hinge domain. The X-ray structure of the mouse hinge domain is shown (PDB: 2WD5), with SMC3 in green and SMC1 in yellow. Residues in between which the YBBR-tag and the FRB-domain were inserted are shown as red and blue spheres, respectively.
B.) Cartoon illustration showing the positions of the YBBR-tags in the hinge-bending sensor constructs used in Figure 5.
C.) Purity and labeling of the hinge-bending sensor constructs used in Figure 5. CY3: donor fluorescence. CY5: acceptor fluorescence.
D.) Example FRET traces of hinge-bending sensor pairs 1, 2 and 3 in the presence of ATP and NIPBL.
E.) Example FRET traces of hinge bending sensor pairs 4 and 5 in the presence of ATP and NIPBL.
F.) Coupled ATPase assay of wild-type and hinge-bending sensor cohesin complexes (sensor pair 1).
G.) Quantification of ATPase rates under different conditions as performed under F.). A 75 base pair (bp) DNA fragment was used as the DNA substrate. Shown are means ± SD and values of individual replicates.
H.) FRET distributions of hinge-bending sensor pair 1 in presence of ATP and NIPBL and in presence or absence of either 75 bp DNA or puc19 plasmid DNA. N: total number of molecules. Shown are means ± SD (3 replicates).
I.) Purity of cohesinSMC3-intFRB/FKBP-SCC1 and cohesinSMC3-intFRB. Shown is a Coomassie stained gel.
J.) Examples of cohesinSMC3-intFRB/FKBP-SCC1 in presence of DMSO or rapamycin as visualized by rotary shadowing EM. Note that complexes locked with rapamycin appear to be in conformations with aligned upper coiled coils, even though the harsh specimen preparation required for rotary shadowing EM separated the coiled coils in the absence of rapamycin. This observation suggests that the coiled coils are very stably aligned in the bent conformation, perhaps because they are twisted around each other as we observed by HS-AFM (Figures 2B and 2C).
K.) Loop maintenance in presence of rapamycin but absence of NIPBL. Loops were first formed by cohesinFKBP-SCC1/SMC3-intFRB in presence of ATP and NIPBL (step 1) followed by flow-in of rapamycin in presence of ATP but in absence of NIPBL (step 2). Shown are means ± SD (2 replicates).