Abstract
Carriers of a pathogenic/likely pathogenic (P/LP) BRCA1/BRCA2/ATM/PALB2 variant are at increased risk of pancreatic ductal adenocarcinoma (PDAC), yet current guidelines recommend surveillance only for those with a family history of PDAC. We aimed to investigate outcomes of endoscopic ultrasound (EUS)-based PDAC surveillance in BRCA1/BRCA2/ATM/PALB2 carriers without a family history of PDAC. We performed a retrospective analysis of all P/LP BRCA1/BRCA2/ATM/PALB2 carriers who underwent endoscopic ultrasound (EUS) at a tertiary care center. Of 194 P/LP BRCA1/BRCA2/ATM/PALB2 carriers who underwent EUS, 64 (33%) had no family history of PDAC and had at least one EUS for PDAC surveillance. These individuals underwent 143 total EUSs, were predominantly female (72%) and BRCA2 carriers (73%), with the majority having a personal history of cancer other than PDAC (67%). The median age at time of first EUS was 62 years (IQR 53–67 years) and a median of 2 EUSs (IQR 1–3) were performed per patient, with a median of 3 years (IQR 2–4.5 years) between the first and last EUS for those with more than 1 EUS. Pancreatic abnormalities were detected in 44%, including cysts in 27%, and incidental luminal abnormalities in 41%. Eight percent developed a new pancreatic mass or cyst during surveillance, two individuals developed PDAC, and no serious complications resulted from surveillance. After discussion of the risks, limitations, and potential benefits, PDAC surveillance can be considered in BRCA1/BRCA2/ATM/PALB2 carriers without a family history of PDAC, however the effectiveness of PDAC surveillance in this population requires further study.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is currently the fourth most common cause of cancer related death in both men and women (1), and is predicted to be the second leading cause of cancer-related death in the United States in 10 years (2). While the majority of PDAC is considered sporadic, 5–10% of PDACs are related to familial risk (3). Individuals with familial risk include those with an identified pathogenic or likely pathogenic (P/LP) variant in a known PDAC risk gene as well as familial pancreatic cancer without a known genetic risk variant (4). A number of PDAC risk genes have been identified; genes associated with increased breast cancer risk, including BRCA1, BRCA2, ATM, and PALB2, have been of particular interest given the frequency with which P/LP variants are identified in families with PDAC (5). Increased PDAC risk has been demonstrated in carriers of P/LP BRCA1, BRCA2, ATM, and PALB2 across multiple studies (6–13), with BRCA2 often considered to carry the highest risk (6,8,11).
Advanced PDAC is associated with a poor prognosis, with 5-year survival for regional and distant metastatic disease being 12% and 3% respectively (1). Conversely, a recent analysis of SEER data demonstrated that the 5-year survival rate for Stage 1A PDAC nearly doubled from 2004 to 2012, and is now greater than 80% (14). This data highlights the compelling need to develop surveillance strategies to detect PDAC at early stages for those at increased risk including BRCA1, BRCA2, ATM, and PALB2 carriers (15). PDAC surveillance is generally performed through regular imaging of the pancreas with endoscopic ultrasound (EUS) or magnetic resonance imaging (MRI), without definitive evidence showing one imaging modality is superior (16,17). Early surveillance results have been promising, with one large European study of high-risk individuals showing that among CDKN2A carriers with surveillance-detected PDAC, the resection rate was 75%, with a 5-year survival of 24% (18). A long-term study of high-risk individuals in the Unites States, primarily from familial pancreatic cancer families, demonstrated that surveillance-detected cancers were surgically resectable 90% of the time with a 90% 3-year survival (19). However, the effectiveness of PDAC surveillance in P/LP BRCA1, BRCA2, ATM, and PALB2 carriers remains less well characterized, and is predominantly restricted to those with a family history of PDAC (20).
Accumulating data about benefits of surveillance in high-risk individuals has led to recommendations endorsing PDAC surveillance in certain high-risk groups. The recent International Cancer of the Pancreas Screening (CAPS) Consortium recommended that individuals with a P/LP BRCA1, BRCA2, ATM, or PALB2 variant who also have a first-degree relative (FDR) with PDAC are eligible to consider PDAC surveillance starting at age 45–50 (21). Further broadening the family history requirement, more recent guidelines from the National Comprehensive Cancer Network (NCCN) recommend consideration of PDAC surveillance in P/LP BRCA1, BRCA2, ATM, or PALB2 carriers with either a FDR or second-degree relative (SDR) with PDAC (22). Similar criteria requiring a family history of PDAC in BRCA1, BRCA2, ATM, and PALB2 are also proposed by other societies (23,24). Additionally, ongoing multicenter early detection studies, such as the Cancer of the Pancreas Screening-5 (CAPS5) study (25,26) as well as the PanFAM-1 study (27), utilize similar inclusion criteria.
While PDAC surveillance recommendations have focused on carriers of a P/LP variant in BRCA1, BRCA2, ATM, or PALB2 with a family history of PDAC, it remains unclear whether individuals without a family history of PDAC may also potentially benefit. This is especially important as small family size, unknown/incorrect family history, or premature deaths due to other cancers/causes may significantly influence reported family history, and therefore influence whether PDAC surveillance is recommended. In fact, the majority of individuals with PDAC who carry a P/LP BRCA1, BRCA2, ATM, or PALB2 variant did not have a prior known family history of PDAC (28). Therefore, to investigate outcomes of PDAC surveillance for BRCA1, BRCA2, ATM, and PALB2 carriers without a family history of PDAC, we herein describe our institution’s experience with PDAC surveillance in this cohort.
Materials and Methods
This is a retrospective analysis of all individuals with a BRCA1, BRCA2, PALB2, or ATM P/LP variant who had an EUS performed at our tertiary care referral center at the University of Pennsylvania (Philadelphia, PA) between 1/1/2008 and 2/1/2021. This study was approved by the University of Pennsylvania Institutional Review Board and was conducted in accordance with the U.S. Common Rule, and a waiver of informed consent was granted by the Institutional Review Board. At the University of Pennsylvania, BRCA1, BRCA2, PALB2, and ATM carriers are often presented with the option to consider PDAC surveillance, irrespective of family history of PDAC, after an extensive discussion of the risks, potential benefits, and uncertainties about PDAC surveillance.
Individuals with a BRCA1, BRCA2, PALB2, or ATM P/LP variant and those who had undergone an EUS were identified through several mechanisms, including enrollment in a University of Pennsylvania PDAC surveillance study entitled “Preliminary Evaluation of Screening for Pancreatic Cancer in Patients with Inherited Genetic Risk,” the University of Pennsylvania Gastrointestinal Cancer Genetics Program Registry, and the University of Pennsylvania Basser Center Registry. Additional carriers who had undergone EUS were identified through an IRB-approved search of the medical records to identify patients with an appropriate ICD-9/10 diagnostic code (ICD-9: V84.01. ICD-10: Z15.0, Z15.01, Z15.02, Z15.03, Z15.09, Z13.7, Z13.79, Z15.89) as well as an appropriate EUS-related CPT procedure code (CPT 43237, 43231, 43232, 43237, 43238, 43240, 43242, 43253, 43259). All patients identified by these mechanisms had their electronic records manually reviewed to confirm each patient had undergone genetic testing identifying a P/LP BRCA1, BRCA2, PALB2, or ATM variant, or was an obligate carrier of a P/LP BRCA1, BRCA2, PALB2, or ATM variant. Individuals with only a BRCA1, BRCA2, PALB2, or ATM variant of uncertain significance were not included in the analysis. Records were manually reviewed to confirm that each patient had at least 1 EUS performed during the study period, including medical encounters and endoscopy reports from our center, as well as all available data from outside of our institution.
Information collected on the entire cohort included sex, race, genetic testing, and whether there was a family history of PDAC, which was defined as having at least 1 FDR or SDR with PDAC. All individuals had a family history recorded in the electronic medical record, with the majority having been seen in a dedicated cancer genetics clinic where a three-generation family pedigree was obtained by a genetic counselor or cancer genetics focused physician. Individuals who were carriers of a P/LP variant in BRCA1, BRCA2, PALB2, or ATM and who had a family history of PDAC in a FDR or SDR were then excluded, followed by exclusion of individuals who had an EUS performed for a reason other than PDAC surveillance. Of the remaining cohort, information collected included demographics, personal history, family history, and EUS records including findings and complications. Descriptive statistics were presented as medians with associated interquartile ranges (IQRs) or as percentages.
Results
Of 1397 P/LP BRCA1, BRCA2, PALB2, and ATM carriers seen at our institution over the study period, 194 (13%) underwent at least 1 EUS (Figure 1). This cohort was predominantly female (76%) and white (93%), with 65% having a P/LP BRCA2 variant and 21% having a P/LP BRCA1 variant (Supplementary Table 1). Of this group, 104 (54%) had at least one FDR or SDR with PDAC, compared to 244 (17%) of our total cohort of 1397 P/LP BRCA1, BRCA2, PALB2, and ATM carriers. Of the 90 individuals who underwent EUS without a family history of PDAC, 26 (29%) underwent EUS for reasons other than PDAC surveillance, including evaluation of abnormal imaging (n = 20), further evaluation of prior endoscopic findings (n = 2), or for cancer staging (n = 4) (Figure 1).
Figure 1:
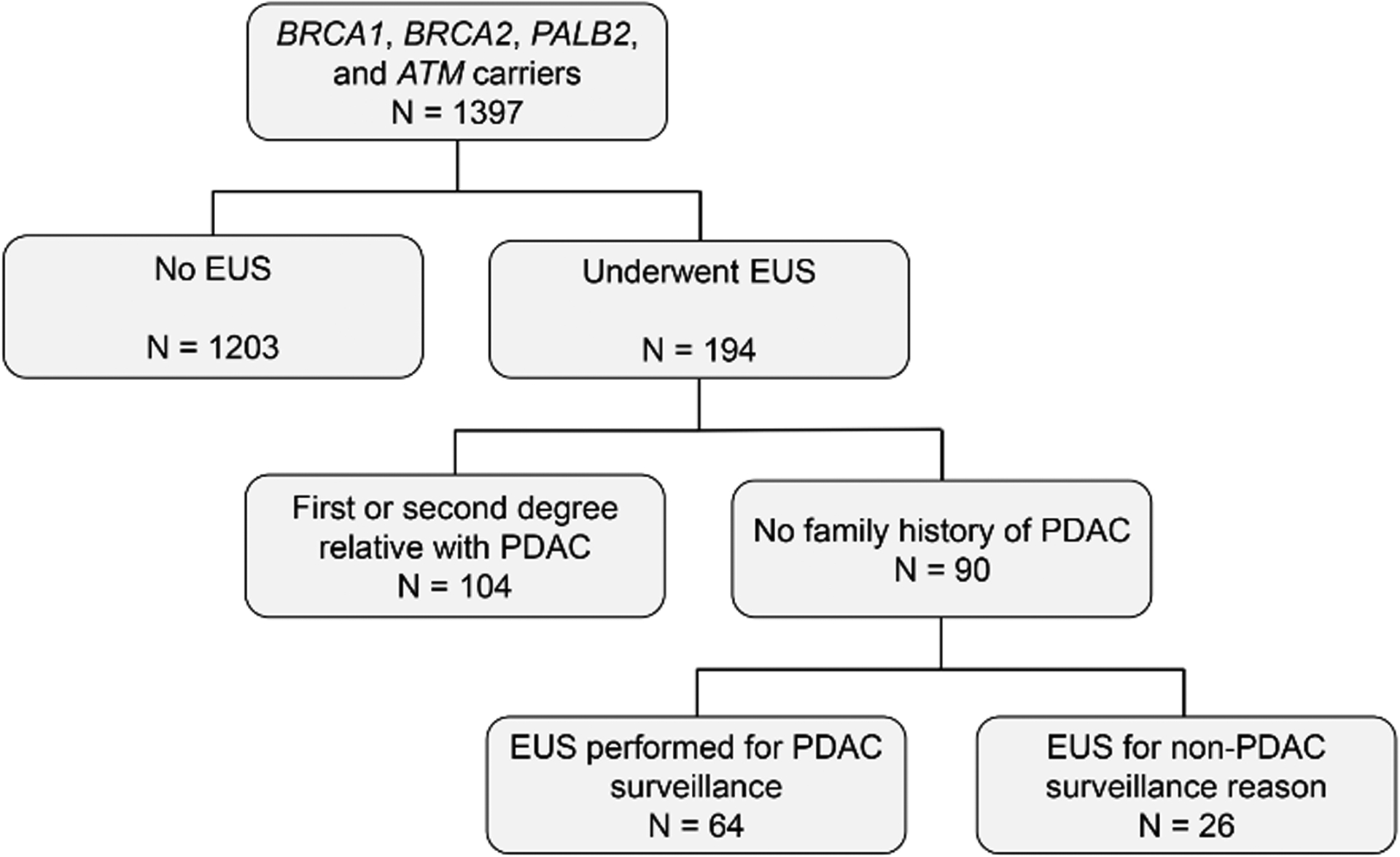
Individuals with a BRCA1, BRCA2, PALB2, or ATM P/LP variant who underwent an EUS.
The remaining 64 P/LP BRCA1, BRCA2, PALB2, and ATM carriers without a family history of PDAC who underwent at least 1 EUS for PDAC surveillance were predominantly female (72%) and white (95%), with 33% having Ashkenazi Jewish ancestry, and with 73% of these individuals had a P/LP BRCA2 variant (Table 1). Twenty-five percent were either current or former smokers, and the majority (73%) reported alcohol use. Three individuals (5%) had a prior history of acute pancreatitis, however, no individuals had a history of chronic pancreatitis or hereditary pancreatitis. Additionally, 6 individuals (9%) had a history of type II diabetes mellitus, and no individuals were reported to have type I diabetes mellitus. Sixty-seven percent of this cohort had a prior cancer, including 34 (54%) with breast cancer. All individuals in the cohort reported a family history of at least one cancer in either an FDR or SDR, including breast cancer in 89%, ovarian cancer in 33%, and prostate cancer in 36%.
Table 1:
Characteristics of P/LP BRCA1, BRCA2, PALB2, and ATM carriers without a family history of PDAC who underwent EUS for PDAC surveillance.
| N = 64 | ||
|---|---|---|
| Age at first EUS, median, IQR | 62 | 53–67 |
| Female | 46 | 72% |
| Race | ||
| White | 61 | 95% |
| Non-white | 2 | 3% |
| Not reported | 1 | 2% |
| Ashkenazi Jewish ancestry | 21 | 33% |
| P/LP gene variant | ||
| BRCA1 | 12 | 19% |
| BRCA2 | 47 | 73% |
| PALB2 | 2 | 3% |
| ATM | 3 | 5% |
| Smoking | ||
| Never | 48 | 75% |
| Former | 13 | 20% |
| Current | 3 | 5% |
| Alcohol use | 47 | 73% |
| Prior pancreatitis | 3 | 5% |
| Type II DM | 6 | 9% |
| Personal history of cancer | ||
| Any cancer | 43 | 67% |
| Breast | 34 | 53% |
| Ovarian | 1 | 2% |
| Prostate | 2 | 3% |
| Family history of cancer | ||
| Any cancer | 64 | 100% |
| Breast | 57 | 89% |
| Ovarian | 21 | 33% |
| Prostate | 23 | 36% |
The 64 P/LP BRCA1, BRCA2, PALB2, and ATM carriers without a family history of PDAC underwent a total of 143 surveillance EUSs. Seven fine needle aspirations (FNAs) were performed; the rate of FNA use was 5%. The median age at first surveillance EUS was 62 (IQR 53–67), and the median number of EUSs performed was 2 (IQR 1–3), with more than one-third of these individuals (35%) having 3 or more EUSs performed (Table 1 and 2). As individual surveillance patterns were highly variable, the median number of years between the first and last EUS recorded for those having 2 or more EUSs was 3 years (IQR 2–4.5 years) (Figure 2). Forty-eight percent (N = 31) of these individuals had other non-EUS imaging of their pancreas with either an MRI or CT scan, with the median number of MRIs/CTs amongst this group being 2 (range 1–3.5) (Supplementary Table 2). Taking these non-EUS pancreatic imaging studies into account, 49 (77%) individuals had more than 1 imaging study of their pancreas performed (Supplementary Table 2).
Table 2:
Number and timing of surveillance EUSs in P/LP BRCA1, BRCA2, PALB2, and ATM carriers without a family history of PDAC.
| N = 64 | |
|---|---|
| Number of EUSs per patient, median, IQR | 2 (1–3) |
| Number of EUSs performed | |
| 1 | 29 (45%) |
| 2 | 13 (20%) |
| 3 | 11 (17%) |
| 4 | 7 (11%) |
| ≥ 5 | 4 (6%) |
| Years between first and last EUS for individuals with ≥ 2 EUS, median (range) | 3 (2–4.5) |
| Years between first and last EUS for individuals with ≥ 2 EUS | |
| 1 | 6 (17%) |
| 2 | 8 (23%) |
| 3 | 9 (26%) |
| 4 | 3 (9%) |
| ≥ 5 | 9 (28%) |
Figure 2:
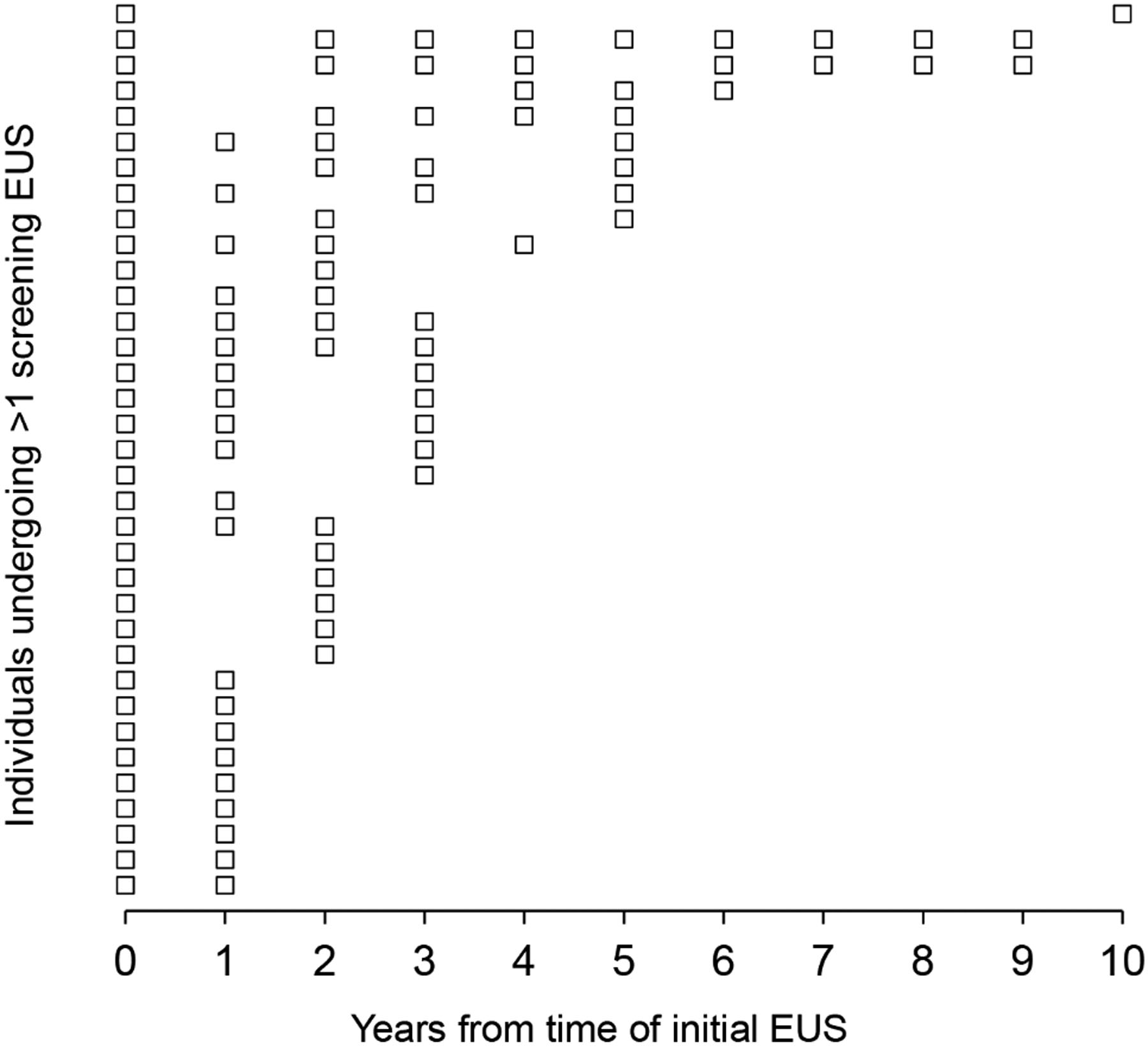
Plot of EUSs performed in P/LP BRCA1, BRCA2, PALB2, and ATM carriers with more than one surveillance EUS. Each row corresponds to one individual’s EUSs starting with the index EUS at year 0.
Amongst the 64 individuals undergoing EUS, 44% had an abnormal pancreatic finding, with pancreatic cysts being most common (27%, Table 3); these were reported as either side-branch intraductal papillary mucinous neoplasms (IPMNs) or uncharacterized pancreatic cysts, with no main duct IPMNs nor mucinous cystic neoplasms identified. Similar results were noted for the 31 individuals who also underwent an MRI or CT surveillance scan, where 42% had an abnormal pancreatic finding that was most often a cyst (32%, Supplementary Table 3). Two individuals (3%) were diagnosed with PDAC during the study period, and although a potential pancreatic mass was noted endosonographically in 3 individuals (5%), only one of these masses turned out to be PDAC. The other two potential masses had nonspecific benign histology on FNA, with one thought to be consistent with a splenule on repeat examination, and the other not being re-visualized on repeat examination. A pancreatic mass or cyst developed during surveillance in 5 individuals (8%) after having a prior normal EUS. Incidental luminal findings were identified in 41% of individuals undergoing EUS, with gastritis (16%), fundic gland polyps (14%), and esophagitis (13%) being most common (Table 3). Important luminal findings such as Barrett’s esophagus, gastric intestinal metaplasia, fundic gland polyp with low grade dysplasia, and Helicobacter pylori infection were also detected incidentally during the surveillance EUSs. The only procedure-related complication reported was a transient post-procedure fever, which resolved without any intervention.
Table 3:
EUS/EGD findings in P/LP BRCA1, BRCA2, PALB2, and ATM carriers.
| Total (N = 64) | % | |
|---|---|---|
| EUS findings | ||
| Any abnormality | 28 | 44% |
| PDAC | 2 | 3% |
| Mass | 3 | 5% |
| Cyst | 17 | 27% |
| Mass/cyst after initial EUS | 5 | 8% |
| Parenchymal abnormality | 10 | 16% |
| Heterogeneity | 4 | 6% |
| Hyperechoic | 2 | 3% |
| Lobularity | 4 | 6% |
| Fatty | 6 | 9% |
| EGD findings | ||
| Any abnormality | 26 | 41% |
| Esophagitis | 8 | 13% |
| Esophageal stricture | 3 | 5% |
| Barrett’s esophagus | 3 | 5% |
| Gastritis | 10 | 16% |
| Gastric ulcer | 2 | 3% |
| Gastric intestinal metaplasia | 3 | 5% |
| Fundic gland polyp* | 9 | 14% |
| Helicobacter pylori | 1 | 2% |
One individual had a fundic gland polyp with low grade dysplasia
Of the two patients who developed PDAC, the first was a female BRCA2 carrier, with a history of a uterine leiomyosarcoma at age 57 and a family history of two paternal cousins with breast cancer. She was a non-smoker with an elevated BMI (37 at the time of diagnosis) who on her initial surveillance EUS at age 58 was found to have a 14 mm hypoechoic cystic lesion in the pancreatic tail that communicated with the main pancreatic duct, but did not directly involve the main pancreatic duct. FNA with cytology revealed evidence of a mucinous cyst with high grade dysplasia (HGD). The patient underwent a distal pancreatectomy and splenectomy, which was the only pancreatic surgery performed in the entire cohort, with pathology revealing a 0.3 cm focus of well-differentiated invasive PDAC arising in association with a side-branch IPMN with HGD, and she remains PDAC-free more than 2 years after surgery. The second patient to develop PDAC was also a female BRCA2 carrier (non-smoker, BMI 25), with no personal history of cancer and a family history of breast cancer in her sister and daughter, who had undergone annual EUS surveillance from ages 72 to 74 with a normal appearing pancreas. The patient did not have re-examination of her pancreas until age 76 where she had an EUS after developing abdominal pain revealing a 4.4 cm × 3.4 cm hypoechoic mass in the uncinate process. FNA was positive for adenocarcinoma with CT scan of the abdomen demonstrating metastatic disease to the liver, and the patient ultimately had a prolonged response to a PARP inhibitor.
Discussion
Carriers of P/LP variants in BRCA1, BRCA2, PALB2, and ATM are at increased risk for PDAC, yet current pancreatic surveillance recommendations advocate for performing PDAC surveillance only if these individuals also have a family history of PDAC. Herein we present the largest report to date of PDAC surveillance with EUS in BRCA1, BRCA2, PALB2, and ATM carriers without a family history of PDAC. We demonstrate that 44% of individuals had an abnormality detected on EUS, including 22% with either a pancreatic mass or cyst. Additionally, 8% developed a new pancreatic mass or cyst during surveillance, and two individuals in our surveillance cohort developed PDAC. Together, these results fill an important knowledge gap in PDAC surveillance in high-risk mutation carriers without a family history of PDAC, which may help guide future surveillance studies and surveillance strategies for this cohort.
Current guidelines for PDAC surveillance in BRCA1, BRCA2, PALB2, and ATM carriers recommend PDAC surveillance only in the setting of a family history of PDAC in a FDR or SDR (21–24). Although the family history requirement may aid in selecting the highest risk individuals, relying on family history alone to determine PDAC surveillance candidates has drawbacks. First, given the other penetrant cancer phenotypes associated with pancreatic risk genes, it is possible that early deaths due to cancers other than PDAC (i.e. breast or ovarian cancer) may limit the number of long-living individuals who develop PDAC. Second, individuals may not know their complete family history, or may have misinformation about the types of cancers present in their families (29,30). Finally, individuals from small families naturally have fewer at-risk relatives. Taken together, these factors may lead to significant underestimation of PDAC risk in some individuals who report no family history of PDAC. This point is further exemplified by our study data, where two BRCA2 carriers without a family history of PDAC subsequently developed PDAC during surveillance. To further address this, the field needs to develop more effective risk prediction tools, allowing more accurate risk determination and a tailored PDAC surveillance approach in all variant carriers regardless of family history. Furthermore, a similar study could also be performed in Lynch syndrome carriers, where the decision to perform surveillance is also tied to the presence of a family history of PDAC (22).
There remains minimal data assessing the uptake of PDAC surveillance among individuals with increased genetic risk, especially in the absence of a family history. We show that among BRCA1, BRCA2, PALB2, and ATM carriers without a family history of PDAC, a robust number of individuals choose to undergo PDAC surveillance, even in the absence of formal guideline-based surveillance recommendations. Over two-thirds of the individuals in this analysis who underwent EUS surveillance had a personal history of cancer, and every individual in the cohort had a family history of cancer. It is possible that this strong personal and family history of cancer influenced individuals’ decisions about whether to proceed with PDAC surveillance. Furthermore, for many of these affected individuals who may have already undergone risk-reducing surgeries, such as bilateral mastectomy and/or salpingo-oophorectomy, the risk of PDAC may be one of the highest cancer risks that this group continues to face, which may also be a strong motivating factor to pursue surveillance. Of additional interest is that the majority of our cohort who underwent surveillance were BRCA2 carriers, suggesting that BRCA2 carriers may have a higher likelihood of wanting to pursue surveillance. Future studies exploring the motivations behind individuals’ decisions to choose to undergo PDAC surveillance, even in the absence of a family history of PDAC, are certainly needed.
In our cohort we found that EUS frequently identified pancreatic abnormalities (44%), including 22% with either masses or cysts, and incidentally discovered luminal abnormalities (40%). These findings are in line other studies, including a multi-center study examining surveillance findings in high-risk individuals demonstrating that 42% had either a pancreatic mass, cyst, or dilated pancreatic duct identified (31). They are similarly comparable to a small series of mutation carriers without a family history of PDAC (32) and a recent meta-analysis of high-risk individuals (17). It is well-known that pancreatic lesions are commonly observed (33), including in the general population (34), and therefore our results are not surprising. However, whether detection of these frequently found pancreatic abnormalities has clinical significance remains uncertain and will require larger and longer-term prospective studies. Furthermore, although not observed with any patients in our cohort, it is possible that pancreatic lesions identified on surveillance may lead to additional work-up and/or surgery, which carries additional potential risk for patients (35).
Eight percent of our cohort developed a pancreatic mass or cystic lesion during surveillance after a prior normal EUS, therefore demonstrating that new lesions can be detected during surveillance. Two individuals (3%) were diagnosed with PDAC, with one with metastatic disease having an EUS 2 years prior to her diagnosis. Similarly, of 97 BRCA1/BRCA2/PALB2/ATM carriers followed by our center with a family history of PDAC and a surveillance EUS performed during the study period, 2 (2%) were diagnosed with an advanced pancreatic lesion (IPMN with HGD). It is possible that had the individual with metastatic disease continued with annual pancreatic imaging this PDAC may have been diagnosed at a resectable stage, and emphasizes the need for close monitoring of individuals who choose to undergo PDAC surveillance, ideally on an annual basis. Additionally, the two PDACs in our surveillance cohort of 62 individuals is a higher frequency than estimates of number-needed-to-screen amongst high-risk cohorts with a family history of PDAC, where between 111–135 high risk patients are predicted to need to be screened to identify a single high-risk lesion (17,36). In addition to the pancreatic findings, other incidentally discovered luminal findings were noted included Barrett’s esophagus, gastric intestinal metaplasia, low grade dysplasia in a fundic gland polyp, and Helicobacter pylori. Treatment and/or follow-up of these luminal findings, which otherwise may not have been detected outside of these surveillance EUSs, may have additional long-term benefits in the patients in whom they were discovered. Alternatively, one could consider performing surveillance EUS in any BRCA1, BRCA2, PALB2, or ATM carrier who is undergoing upper endoscopy for another clinical indication.
There continues to be debate about which modality of PDAC surveillance is most effective, with no consensus as to whether EUS or MRI is preferred (16,31). This has similarly been emphasized in guidelines recommending use of either imaging modality in appropriate high risk individuals (21,22). MRI may be more sensitive for detecting cystic lesions of the pancreas, whereas EUS may be better at detecting solid lesions (16,35). Given that the majority of PDACs develop as solid lesions via the PanIN pathway, rather than from cysts (37), EUS performed by experienced endoscopists may have added benefit when incorporated into PDAC surveillance regimens (35).
There are limitations to our study including our small sample size. Our analysis focusing on carriers without a family history of PDAC limited our sample size to less than a third of the BRCA1, BRCA2, PALB2, and ATM carriers who underwent EUS and less than a tenth of the total number of carriers followed at our institution, thus leading to potential selection bias. Ongoing studies (i.e. CAPS5 (25)) that our institution is participating in are prospectively collecting data on carriers with a family history, and our data on these carriers with a family history will be reported with the collective data of these ongoing studies. Another limitation of our study is that for individuals who underwent more than one surveillance EUS, the intervals between EUSs were highly variable. Additionally, given the retrospective nature of this study and the inherent selection bias, it is possible that there is over-estimation of the rate of pancreatic findings and PDAC. A final limitation of our study is the reporting of family history, which in most cases was by self-report. However, the majority of these patients were seen in a dedicated cancer genetics clinic where a three-generation family pedigree was obtained by a genetic counselor or cancer genetics focused physician.
PDAC surveillance is an important and evolving area of cancer risk management, yet limitations and uncertainties highlight the need for continued research in the field (38). The downsides to PDAC surveillance include discovery of false positive findings with potential for unnecessary intervention (39), the small but tangible risks associated with surveillance procedures, increased health care costs (40), as well as lack of large-scale data showing surveillance is conclusively effective for preventing death from PDAC. However, at this time PDAC surveillance provides the only option for patients at increased risk to detect PDAC early and potentially allow it to be treated surgically. In the past, surveillance of BRCA1, BRCA2, PALB2, and ATM carriers has been largely governed by family history; given the limitations of family history-based surveillance outlined earlier, whether this is the optimal strategy for PDAC surveillance remains to be determined. Our data illustrates that PDAC surveillance with EUS might be considered in all carriers of a P/LP variant in BRCA1, BRCA2, PALB2, or ATM, regardless of family history, with further study of surveillance outcomes in this population being needed. However, prior to consideration of PDAC surveillance initiation, it is critical for providers to discuss the risks and potential benefits, as well as the limitations of PDAC surveillance, to allow patients to make a well-informed choice.
Supplementary Material
Prevention Relevance:
BRCA1/BRCA2/ATM/PALB2 carriers have increased pancreatic ductal adenocarcinoma (PDAC) risk, yet are typically not eligible for PDAC surveillance in the absence of PDAC family history. Herein we describe outcomes of PDAC surveillance in BRCA1/BRCA2/ATM/PALB2 carriers without a family history of PDAC, showing that PDAC surveillance can be considered in this high-risk group.
Acknowledgements
NIH/NIDDK grant R03DK120946 (B Katona), NIH/NCI grant U01CA210170 (B Katona, A Rustgi), Smith Family Research Fund (B Katona), and the Basser Center for BRCA (S Domchek).
Footnotes
Disclosures: BWK: Consulting (Exact Sciences), Travel (Janssen). ELC: Travel (Imedex, Foundation Medicine, AstraZeneca), Research Funding (Merck, Janssen, and Becton Dickinson). SMD: Honoraria (AstraZeneca, Clovis, Bristol-Myers Squibb). MLK: Equity (Virgo Systems, Dark Canyon Labs), Consultant (Medtronic, BSC, Olympus), DSMB (Applied Clinical Intelligence, Shionogi)
References
- 1.American Cancer Society. Cancer Facts & Figures 2020. Atlanta: American Cancer Society; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74(11):2913–21 doi 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Chaffee KG, Oberg AL, McWilliams RR, Majithia N, Allen BA, Kidd J, et al. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genet Med 2018;20(1):119–27 doi 10.1038/gim.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood LD, Yurgelun MB, Goggins MG. Genetics of Familial and Sporadic Pancreatic Cancer. Gastroenterology 2019;156(7):2041–55 doi 10.1053/j.gastro.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Dudley B, Brand RE. Pancreatic Cancer Surveillance: Who, When, and How. Curr Treat Options Gastroenterol 2019;17(4):681–91 doi 10.1007/s11938-019-00247-0. [DOI] [PubMed] [Google Scholar]
- 6.Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018;319(23):2401–9 doi 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhen DB, Rabe KG, Gallinger S, Syngal S, Schwartz AG, Goggins MG, et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med 2015;17(7):569–77 doi 10.1038/gim.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, Singletary C, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015;121(2):269–75 doi 10.1002/cncr.29041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iqbal J, Ragone A, Lubinski J, Lynch HT, Moller P, Ghadirian P, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. Br J Cancer 2012;107(12):2005–9 doi 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst 2002;94(18):1365–72 doi 10.1093/jnci/94.18.1365. [DOI] [PubMed] [Google Scholar]
- 11.Moran A, O’Hara C, Khan S, Shack L, Woodward E, Maher ER, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Fam Cancer 2012;11(2):235–42 doi 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 12.Roberts NJ, Jiao Y, Yu J, Kopelovich L, Petersen GM, Bondy ML, et al. ATM mutations in patients with hereditary pancreatic cancer. Cancer Discov 2012;2(1):41–6 doi 10.1158/2159-8290.CD-11-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Leslie G, Doroszuk A, Schneider S, Allen J, Decker B, et al. Cancer Risks Associated With Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J Clin Oncol 2020;38(7):674–85 doi 10.1200/JCO.19.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blackford AL, Canto MI, Klein AP, Hruban RH, Goggins M. Recent trends in the incidence and survival of Stage 1A Pancreatic Cancer: A Surveillance, Epidemiology, and End Results analysis. J Natl Cancer Inst 2020. doi 10.1093/jnci/djaa004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singhi AD, Koay EJ, Chari ST, Maitra A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019;156(7):2024–40 doi 10.1053/j.gastro.2019.01.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harinck F, Konings IC, Kluijt I, Poley JW, van Hooft JE, van Dullemen HM, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2016;65(9):1505–13 doi 10.1136/gutjnl-2014-308008. [DOI] [PubMed] [Google Scholar]
- 17.Kogekar N, Diaz KE, Weinberg AD, Lucas AL. Surveillance of high-risk individuals for pancreatic cancer with EUS and MRI: A meta-analysis. Pancreatology 2020. doi 10.1016/j.pan.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 18.Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthai E, Carrato A, et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol 2016;34(17):2010–9 doi 10.1200/JCO.2015.64.0730. [DOI] [PubMed] [Google Scholar]
- 19.Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A, et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018;155(3):740–51 e2 doi 10.1053/j.gastro.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saldia A, Olson SH, Nunes P, Liang X, Samson ML, Salo-Mullen E, et al. Outcome of Pancreatic Cancer Surveillance Among High-Risk Individuals Tested for Germline Mutations in BRCA1 and BRCA2. Cancer Prev Res (Phila) 2019;12(9):599–608 doi 10.1158/1940-6207.CAPR-18-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, et al. Management of patients with increased risk for familial pancreatic cancer: updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020;69(1):7–17 doi 10.1136/gutjnl-2019-319352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daly MB, Pilarski R, Yurgelun MB, Berry MP, Buys SS, Dickson P, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J Natl Compr Canc Netw 2020;18(4):380–91 doi 10.6004/jnccn.2020.0017. [DOI] [PubMed] [Google Scholar]
- 23.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: Genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 2015;110(2):223–62; quiz 63 doi 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoffel EM, McKernin SE, Brand R, Canto M, Goggins M, Moravek C, et al. Evaluating Susceptibility to Pancreatic Cancer: ASCO Provisional Clinical Opinion. J Clin Oncol 2019;37(2):153–64 doi 10.1200/JCO.18.01489. [DOI] [PubMed] [Google Scholar]
- 25.The Cancer of the Pancreas Screening-5 (CAPS5) Study. ClinicalTrials.gov, NCT02000089. https://clinicaltrials.gov/ct2/show/NCT02000089.
- 26.Katona BW, Mahmud N, Dbouk M, Ahmad N, Chhoda A, Dudley B, et al. COVID-19 related pancreatic cancer surveillance disruptions amongst high-risk individuals. Pancreatology 2021. doi 10.1016/j.pan.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.A Study of IMMray™ PanCan-d Test for Early Detection of Pancreatic Cancer in High-risk Groups. ClinicalTrials.gov, NCT03693378. https://clinicaltrials.gov/ct2/show/NCT03693378.
- 28.Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, et al. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. J Clin Oncol 2017;35(30):3382–90 doi 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sijmons RH, Boonstra AE, Reefhuis J, Hordijk-Hos JM, de Walle HE, Oosterwijk JC, et al. Accuracy of family history of cancer: clinical genetic implications. Eur J Hum Genet 2000;8(3):181–6 doi 10.1038/sj.ejhg.5200441. [DOI] [PubMed] [Google Scholar]
- 30.Douglas FS, O’Dair LC, Robinson M, Evans DG, Lynch SA. The accuracy of diagnoses as reported in families with cancer: a retrospective study. J Med Genet 1999;36(4):309–12. [PMC free article] [PubMed] [Google Scholar]
- 31.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012;142(4):796–804; quiz e14–5 doi 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thiruvengadam SS, Chuang J, Huang R, Girotra M, Park WG. Chronic pancreatitis changes in high-risk individuals for pancreatic ductal adenocarcinoma. Gastrointest Endosc 2019;89(4):842–51 e1 doi 10.1016/j.gie.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Megibow AJ, Baker ME, Morgan DE, Kamel IR, Sahani DV, Newman E, et al. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J Am Coll Radiol 2017;14(7):911–23 doi 10.1016/j.jacr.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010;105(9):2079–84 doi 10.1038/ajg.2010.122. [DOI] [PubMed] [Google Scholar]
- 35.Overbeek KA, Levink IJM, Koopmann BDM, Harinck F, Konings I, Ausems M, et al. Long-term yield of pancreatic cancer surveillance in high-risk individuals. Gut 2021. doi 10.1136/gutjnl-2020-323611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corral JE, Mareth KF, Riegert-Johnson DL, Das A, Wallace MB. Diagnostic Yield From Screening Asymptomatic Individuals at High Risk for Pancreatic Cancer: A Meta-analysis of Cohort Studies. Clin Gastroenterol Hepatol 2019;17(1):41–53 doi 10.1016/j.cgh.2018.04.065. [DOI] [PubMed] [Google Scholar]
- 37.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet 2011;378(9791):607–20 doi 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henrikson NB, Aiello Bowles EJ, Blasi PR, Morrison CC, Nguyen M, Pillarisetty VG, et al. Screening for Pancreatic Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2019;322(5):445–54 doi 10.1001/jama.2019.6190. [DOI] [PubMed] [Google Scholar]
- 39.Canto MI, Goggins M, Yeo CJ, Griffin C, Axilbund JE, Brune K, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol 2004;2(7):606–21 doi 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 40.Corral JE, Das A, Bruno MJ, Wallace MB. Cost-effectiveness of Pancreatic Cancer Surveillance in High-Risk Individuals: An Economic Analysis. Pancreas 2019;48(4):526–36 doi 10.1097/MPA.0000000000001268. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


