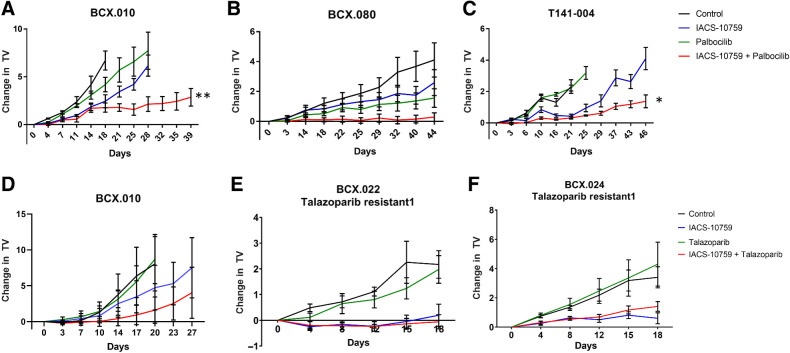
Figure 6.
Validation of functional shRNA screen to identify potential IACS-10759 combination partners. A, We tested palbociclib (50 mg/kg daily) in BCX.010 PDX in vivo. The combination of IACS-10759 and palbociclib showed clear improvement over both single agents similar to in vitro. B, We further validated palbociclib + IACS-10759 in an additional retinoblastoma gene positive less sensitive TNBC PDX (BCX.080). Data are shown as mean ± SEM. **, P < 0.001. C, We also tested palbociclib + IACS-10759 using a PDX (T141–003) generated from a patient with breast cancer who had received palbociclib and progressed. Data are shown as mean ± SEM. *, P < 0.01. D, We tested talazaparib (0.3 mg/kg daily) + IACS-10759 combination in BCX.010 PDX in vivo but found with a more limited combination efficacy. E and F, We next tested talazoparib in combination with IACS-10759 in PDXs with acquired resistance to talazoparib (BCX.022 talazoparib resistant 1 and BCX.024 talazoparib resistant 1). These models were created by treating talazoparib–sensitive PDXs until tumors were not palpable and then collecting and serial passaging the grown tumors. The combination did not have improved efficacy in these models, but IACS-10759 did have significantly greater inhibition versus talazoparib (BCX.022 talazoparib resistant, P < 0.001; BCX.024 talazoparib resistant, P < 0.01).