Figure 5. Activation of PERK/eIF2α signaling inhibits protein synthesis.
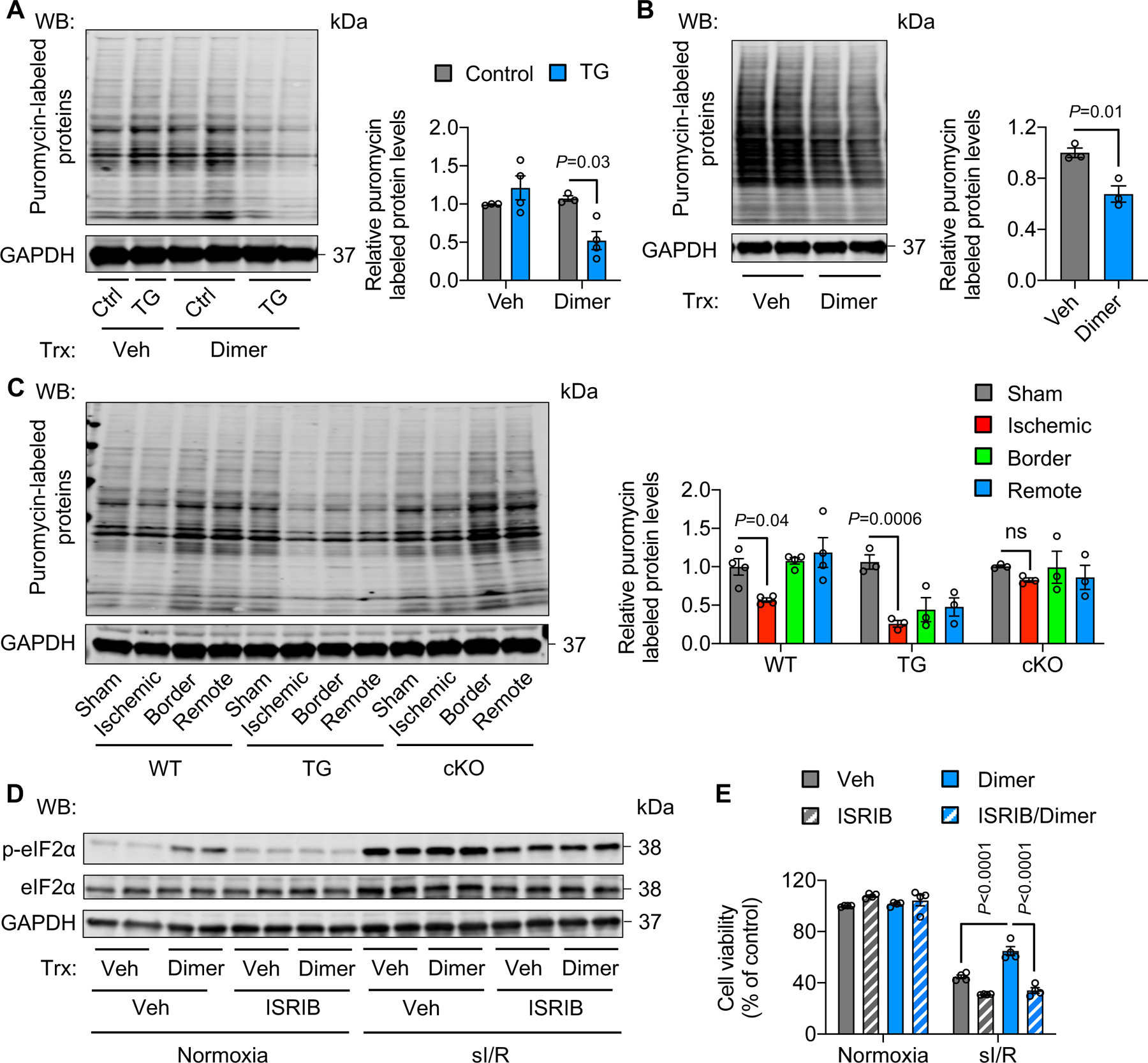
A. PERK activation by dimer in PERK TG mice decreased protein synthesis. Dimer was injected to TG mice for 2 hours. Approximately 30 minutes before heart collection, puromycin was administrated to label newly synthesized proteins. n=3.
B. Activation of PERK in H9c2 cells reduced protein synthesis. Adenoviruses expressing FKBP-PERK were used to infect H9c2 cells, which were then treated with dimer for 30 minutes. Puromycin was added to label newly synthesized proteins 15 minutes before cell harvest. n=3.
C. PERK TG mice showed a lower level of protein synthesis after I/R in comparison to wild-type controls. In contrast, PERK cKO prevented the decrease of protein synthesis. n=3 to 4.
D. ISRIB, an eIF2α phosphorylation inhibitor, suppressed eIF2α phosphorylation in PERK-activated H9c2 cells.
E. ISRIB treatment abolished the protective effect of PERK under sI/R. n=3.
Two-way ANOVA was conducted, followed by Tukey’s multiple comparisons test (A, E). Unpaired Student’s t test was conducted (B). One-way ANOVA was conducted, followed by Tukey’s multiple comparisons test (C). Data are represented as mean±SEM. WB, Western blot; WT, wild-type; TG, transgenic; cKO, cardiac-specific knockout; not significant; Veh, vehicle; and sI/R, simulated ischemia/reperfusion.
