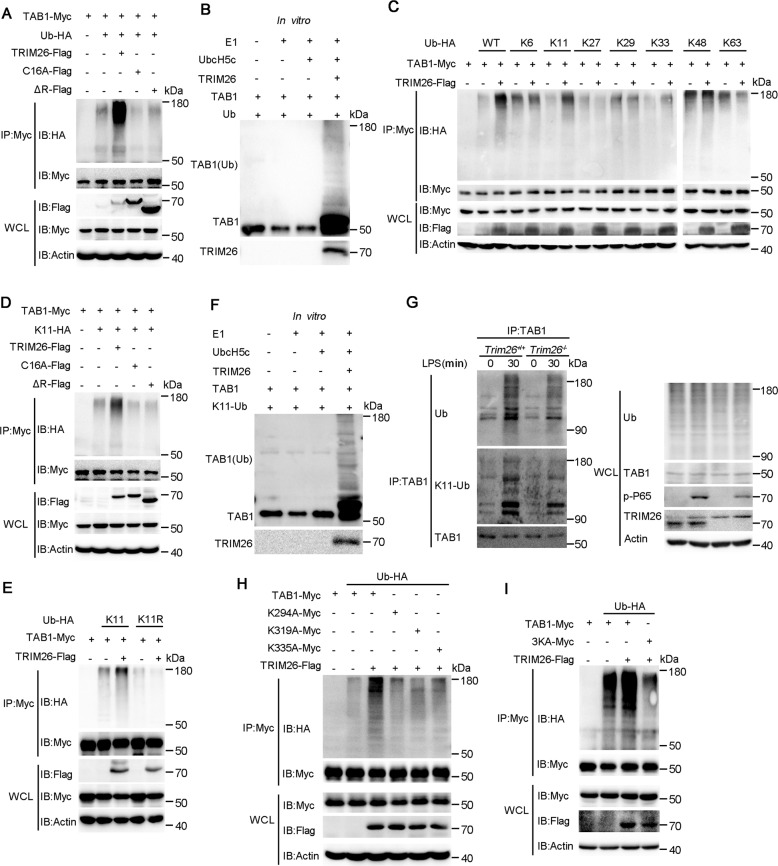
Fig. 4. TRIM26 targets Lys294, 319, and 335 of TAB1 for K11-linked polyubiquitination.
A Immunoprecipitation analysis of the TAB1 ubiquitination in HEK293T cells transfected with plasmids encoding Myc-TAB1, HA-ubiquitin (WT) together with a control vector or plasmids encoding Flag-TRIM26(WT) or Flag-TRIM26 (C16A) or Flag-TRIM26(ΔR). B Recombinant TRIM26 and TAB1 proteins were prepared in an in vitro transcription and translation system. In vitro ubiquitination assay was performed in the presence of Ub, E1, UbcH5c, TRIM26, and TAB1. The ubiquitination of TAB1 was examined by immunoblot analysis with anti-TAB1 Ab. C The HEK293T cells were transfected with plasmids of Flag-TRIM26 and Myc-TAB1 together with HA-Ub or its mutants for 24 h and then followed by co-immunoprecipitation with anti-Myc Ab and immunoblotting analysis with anti-HA Ab. D Co-immunoprecipitation analysis of the ubiquitination of TAB1 in HEK293T cells transfected with plasmids encoding Myc-TAB1, HA-ubiquitin(K11) mutant, or HA-ubiquitin(K11R) mutant together with a control vector or plasmids encoding Flag-TRIM26(WT) or Flag-TRIM26 (C16A) or Flag-TRIM26(ΔR). E Co-immunoprecipitation analysis of the ubiquitination of TAB1 in HEK293T cells transfected with plasmids encoding Myc-TAB1, HA-ubiquitin(K11) mutant, or together with a control vector or plasmids encoding Flag-TRIM26. F In vitro ubiquitination assay was performed in the presence of K11-Ub, E1, UbcH5c, TRIM26, and TAB1. The ubiquitination of TAB1 was examined by immunoblot analysis with anti-TAB1 Ab. G Lysates from Trim26+/+ and Trim26–/– mice peritoneal macrophages stimulated with LPS (200 ng/ml) for the indicated times were subjected to immunoprecipitation with anti-TAB1 Ab followed by immunoblot analysis with anti-ubiquitin Ab. H Co-immunoprecipitation analysis of the polyubiquitination of TAB1 WT and its mutants, in which the lysine residues at position 294, 319, 335 were replaced with alanine individually, in HEK293T cells transfected with plasmids encoding Myc-TAB1 WT or its mutants, plus Flag-TRIM26 and HA-ubiquitin. I Co-immunoprecipitation analysis of the polyubiquitination of TAB1 WT and its mutant 3KA, in which all the three lysine residues at position 294, 319, 335 were replaced with alanine, in HEK293T cells transfected with plasmids encoding Myc-TAB1 WT or its mutants, plus Flag-TRIM26 and HA-ubiquitin. Similar results were obtained in three independent experiments.