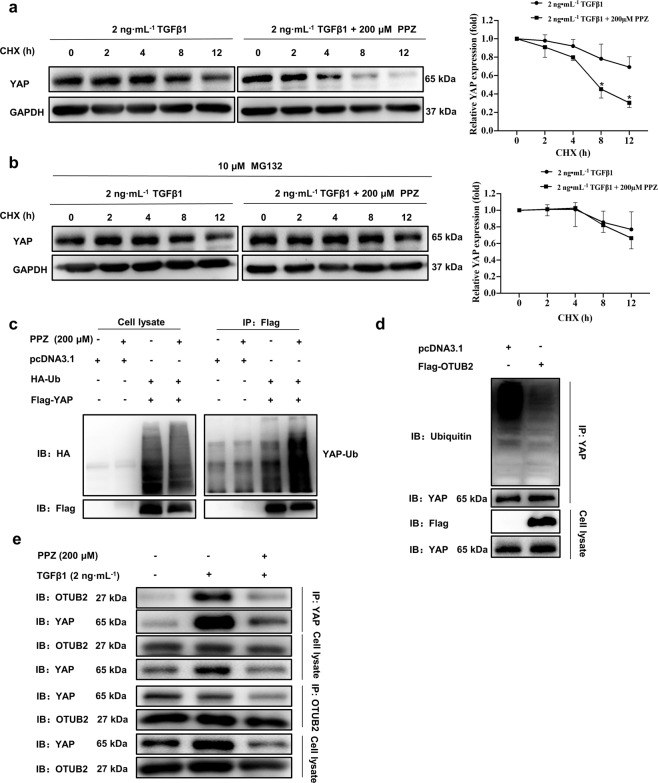
Fig. 7. PPZ promoted the proteasome-dependent degradation and ubiquitination of YAP.
a LX-2 cells were starved for 24 h and then treated with TGF-β1 (2 ng· mL−1) and CHX (20 µg· mL−1) with or without PPZ (200 µM) for the indicated times. The protein expression of YAP was measured by Western blotting. b LX-2 cells were treated with TGF-β1 (2 ng· mL−1), CHX (20 µg ·mL−1) and MG132 (10 µM) with or without PPZ (200 µM) for the indicated times. Changes in the protein levels of YAP are shown. For a, b, the data are presented as the mean ± SD of three independent experiments. *P < 0.05 vs. the 2 ng· mL−1 TGF-β1 group. c HEK293T cells were transfected with Flag-YAP (2.5 µg) and HA-Ubiquitin (2.5 µg) plasmids and subsequently treated with PPZ. Whole-cell extracts were immunoprecipitated with anti‐Flag and blotted with an anti‐HA antibody. d The ubiquitination of endogenous YAP in LX-2 cells transfected with Flag-OTUB2 (2.5 µg) or vector. e The interaction between YAP and OTUB2 in LX-2 cells was investigated using immunoprecipitation assay.