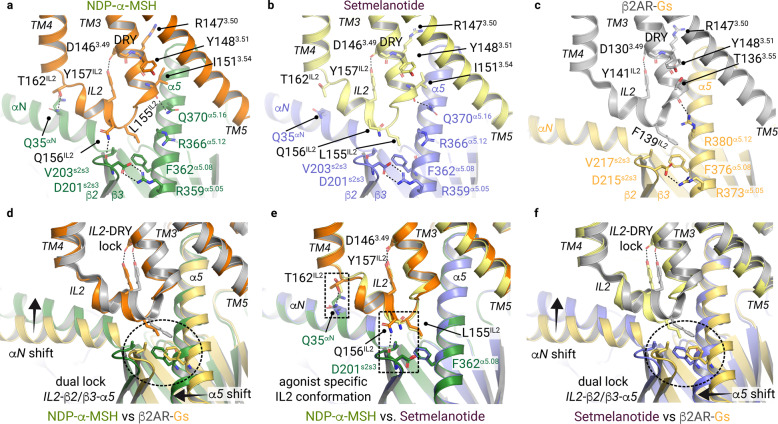
Fig. 7. Gs-protein adjustment at IL2.
a–c Display of the binding interface between TM3–TM5 and Gs-protein in the NDP-α-MSH (a), setmelanotide (b) bound MC4R–Gs complexes, and the β2AR–Gs complex (PDB ID: 3sn6) (c). Interactions of IL2 and TM3 are displayed as black dashed lines. d–f The superposition of NDP-α-MSH–MC4R–Gs and setmelanotide–MC4R–Gs complexes with the β2AR–Gs complex display the IL2–β2/β3–α5 lock of L155 IL2 (MC4R) with V203 and F362 (Gαs), as well as F139 IL2 (β2AR) with V217 and F376 (Gαs) adjusting the position of the α5 helix. A relative α5 shift can be noticed, which results in a slight rotation of the entire Gs coupled to MC4R compared to that coupled to β2AR, most prominently visible by an αN helix shift. e Superposition of both agonist-bound MC4R–Gs structures highlight changes of the hydrogen bonds between Gαs and IL2 residues (dashed boxes).