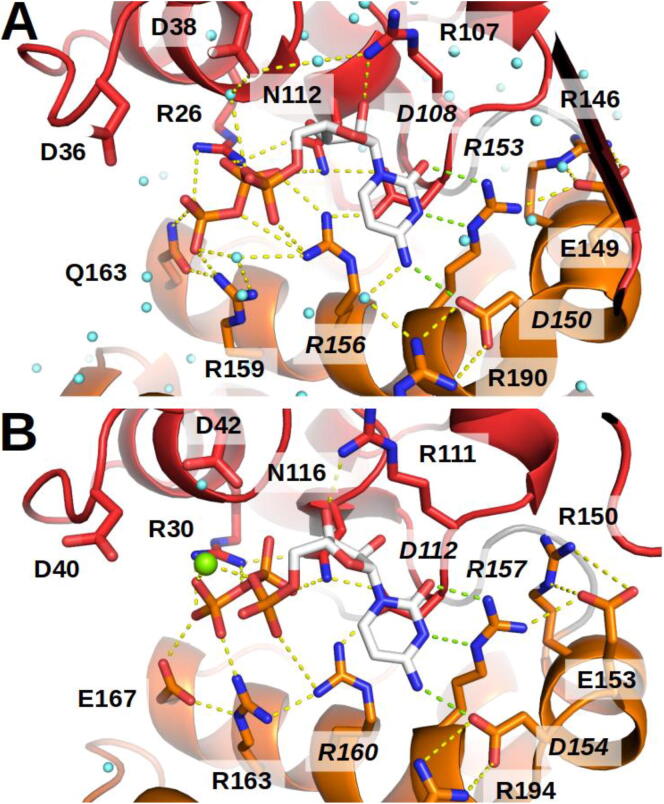
Fig. 4.
A highly conserved CTP binding pocket. Close-up views of the catalytic core of PhaCCA (A) and GstCCA (B) in the presence of CTP. Highly conserved residues with side chains contacting the nucleotide are indicated. Water molecules are represented as blue spheres and magnesium ion bound to GstCCA as a green sphere. No magnesium site could be identified in PhaCCA structure. The dense network of interactions (with a distance < 4 Å) between protein side chains and the substrate is symbolized by dashed lines. Four side chains indicated in italics interact directly with the nucleobase and constitute the amino acid template in motif D: two couples of Arg/Asp residues, one forming a stacking platform below the base, the second making three hydrogen bonds indicated by green dashed lines with the Watson-Crick face of CTP. The latter residues are hold in place by additional connections to surrounding side chains. In both structures, the orientation of the CTP is almost identical and the amino acid template of motif D forms the same interactions to the bound base moiety, indicating that the templating function is not specifically cold adapted in PhaCCA. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)