Abstract
Purpose
InnoSEAL Plus is an adhesive, coagulant-free hemostatic material that mimics the adhesion mechanism of marine mussels. This study reports on the safety and efficacy of InnoSEAL Plus for patients with hemorrhage after hepatectomy despite first-line hemostasis treatments.
Methods
This is a multicenter, prospective, single-blinded, randomized clinical trial involving 96 hepatectomy patients. TachoSil was used as a comparator group. Three-minute and 10-minute hemostatic success rates were monitored. Rebleeding rates were also observed. Safety was assessed by recording all novel undesirable symptoms.
Results
InnoSEAL Plus showed a 3-minute hemostasis rate of 100%, while TachoSil had a rate of 98.0% (48 of 49 patients), demonstrating that the 2 had similar hemostatic efficacies. The difference in efficacy between the test and comparator group was 2.04%, and the lower limit of the one-sided 97.5% confidence interval was −1.92%; as this is greater than the noninferiority limit of −23.9%, the 2 treatments were equivalent. Meanwhile, the 10-minute hemostatic success rate was the same in both groups (100%). No rebleeding occurred in either group. In the safety evaluation, 89 patients experienced adverse events (45 in the test group and 44 in the comparator group). The difference between the 2 groups was not significant. No death occurred after application of the test or comparator group product.
Conclusion
Given that InnoSEAL Plus is a coagulation factor-free product, the hemostasis results are encouraging, especially considering that TachoSil contains a coagulation factor. InnoSEAL Plus was found to be a safe and effective hemostatic material for control of bleeding in hepatectomy patients.
Keywords: Clinical trial, Hemostatics, Hepatectomy, Mussel adhesion, Validation study
INTRODUCTION
Hemostasis during surgery is critical for surgical success. Blood loss during surgery is known to be a predictor of morbidity and mortality [1,2]. Although the use of advanced surgical procedures and techniques has greatly reduced blood loss during various types of surgery, intraoperative and postoperative bleeding are still common in patients undergoing hepatic resection [3].
The management of hemorrhage during liver resection can be especially challenging. Hepatosinusoidal structures lack the capacity for vasocontraction due to the absence of smooth muscles. For this reason, persistent bleeding often occurs on hepatic resection surfaces. Many surgical techniques have been developed to control bleeding in patients undergoing hepatectomy. Bleeding from vessels can be managed by primary surgical methods such as sutures, ligatures, clipping, etc. In addition, various hemostatic agents are widely applied to control surgical bleeding when standard techniques are insufficient.
Hemostatic agents can be divided into 2 categories; those that contain a coagulation factor to directly promote hemostasis (biologic agents), and those that activate the coagulation cascade through various mechanisms (physical agents). In general, biological agents that promote clot formation by triggering coagulation cascades are preferred over physical agents [4]. Of these hemostatic agents, fibrin sealants are increasingly used for hemostasis during liver resection. TachoSil (Takeda Austria GmbH, Wien, Austria) is a representative biological agent-based product [5].
An alternative method that rapidly triggers blood coagulation has been studied by researchers in the field of biomaterial science. Marine mussels form holdfasts on the surfaces of rocks, wood, metals, and various synthetic polymers. A polyphenolic catechol residue from 3,4-dihydroxy-L-phenylalanine (DOPA) and an amine residue from lysine synergistically act to promote strong mussel adhesion even in the presence of water [6,7]. Recently, we reported that a mussel-mimetic polymer containing catechol and amine groups as adhesion components exhibited serum protein aggregation upon blood contact [8]. The phenomenon observed upon contact between mussel-inspired adhesive and blood is very similar to fibrin-mediated coagulation. Importantly, no biological agent such as fibrin is necessary in this alternative artificial coagulation process. Also, a decrease in the rate of complications such as thromboembolic events is expected with use of the novel product.
The aims of this study were to demonstrate noninferiority in efficacy of the mussel-inspired hemostatic material, InnoSEAL Plus (InnoTherapy, Seoul, Korea), compared with TachoSil in Korean patients undergoing liver resection and to assess and compare the safety of the 2 products in these patients.
METHODS
Patients
This study was a multicenter, single-blinded clinical trial in hepatectomy patients with a comparator group, TachoSil. The potential subjects included patients who were over 19 years old and were scheduled for hepatectomy due to hepatocellular carcinoma, intrahepatic cholangiocarcinoma, intrahepatic cholelithiasis, metastatic liver cancer, or donation for a liver transplant at participating hospitals (Pusan National University Hospital, Pusan National University Yangsan Hospital, Samsung Medical Center, and National Cancer Center).
Patients were excluded if significant major bleeding was experienced despite first-line hemostasis treatments. Other exclusions included a history of hepatic cirrhosis, presence of hepatic portal hypertension, history of serious coagulation disorders (international normalized ratio > 2), emergency surgery, immunodeficiency, bilirubinemia (> 2.5 mg/dL), history of liver transplantation or severe postoperative complication, allergies to blood products or chitosan, alcohol or drug abuse, pregnancy or breastfeeding, or participation in other clinical trials in the past 1 month.
The potential subjects of this clinical trial underwent hepatic resection by open or laparoscopic surgery according to the institution’s standard procedure on the scheduled operation day. If following hepatic resection, bleeding in the form of oozing persisted in the resection margin after hemostasis by a primary hemostatic method (suture, ligation, vascular clip, argon beam coagulation, electrocautery, etc.), the patients were finally registered and randomized, and hemostasis was performed using either the experimental or comparator product. The trial took place from November 2017 to September 2018, and the total number of enrolled patients was 96.
This study was approved by the Ministry of Food and Drug Safety of South Korea (approval No. 796) and the Institutional Review Boards of the participating hospitals (Pusan National University Hospital, No. D-1709-016-058; Pusan National University Yangsan Hospital, No. 03-2017-015; Samsung Medical Center, No. SMC 2017-08-080; National Cancer Center, No. NCC2017-0286). All patients voluntarily signed an informed consent form.
Sample size calculation and rationale
In this clinical trial, the hemostasis efficacy of InnoSEAL Plus, the test material, was compared with that of TachoSil, the comparator group material. The primary efficacy endpoint was hemostatic success 3 minutes after the material was applied (i.e., 3-minute success). The purpose of the clinical efficacy test is to demonstrate that InnoSEAL Plus is not clinically inferior to TachoSil, a comparator group product. The material was applied to patients who continued bleeding in the form of oozing despite the use of primary hemostatic methods during hepatic resection. To determine the minimum number of patients (i.e., the sample size), N, the following calculation was performed.
where Ho: Pt ≤ Pc − δ vs. Ha: Pt > Pc – δ
Pt: 3-minute hemostatic success rate of the test group
Pc: 3-minute hemostatic success rate of the comparator group
δ: Noninferiority margin (23.9%)
As Pt is the success rate, which can only be obtained after completion of the clinical study, it was necessary to use a previously published value to calculate the sample size, N. We used 0.807 based on the hemostasis success rate reported by Genyk et al. [9]. The maximum clinically acceptable difference in efficacy between the test material and the comparator product, known as the ‘noninferiority margin,’ was set to 23.9%.
Based on these values, the number of subjects in each group calculated by the formula below, assuming a one-sided significance level of 0.025 and power of 80%, was about 43. Considering 10% dropout, we set out to enroll 48 subjects in each group, for a total of 96 subjects in this clinical trial.
Randomization
Patients who satisfied all inclusion/exclusion criteria were finally enrolled in this clinical trial. They were randomly assigned to either the test group or the comparator group. The randomization ratio (test group to comparator group) was set at 1:1. The permuted block randomization method was applied for each study institution, and a randomization code was generated with SAS Proc Plan (SAS Institute, Cary, NC, USA). The seed number was randomly selected by the person in charge of random assignment.
A randomized envelope was produced and delivered to the investigator for each subject. This envelope was opened at the time of randomization to maintain the security of the randomization code for future subjects.
Surgical techniques and efficacy outcomes
Patients with persistent oozing hemorrhage from transected liver surfaces after hepatectomy despite first-line hemostasis (suture, ligation, vascular clips, argon beam coagulation, or electrocautery) were finally enrolled in this study, and each was assigned a subject enrollment number. The test material (InnoSEAL Plus) or the comparator (TachoSil) was applied to all oozing sites. The test and comparator hemostatic materials were larger than 1–2 cm in the X/Y dimension at the site of hemorrhage. A mild pressure was used to apply the hemostatic materials for 3 minutes. To assess the efficacy of the test material, time for hemostasis was measured; 3-minute hemostasis (primary outcome) and 10-minute hemostasis (secondary outcome) were observed for all enrolled patients. The primary efficacy success rate was calculated by the proportion of subjects showing successful 3-minute hemostasis divided by the total number of patients who received either InnoSEAL Plus or TachoSil. The same calculations were performed for the secondary efficacy success rate and the rebleeding rate.
In general, the subjects remained hospitalized for 7 days for postoperative monitoring, but the precise time of hospital discharge was determined at the discretion of the investigator according to the institution’s standard practices. The subject visited the institution on postoperative day (POD) 30 for a follow-up safety assessment. Each patient’s participation in the study ended either at the 30-day follow-up visit if there were no adverse events (AEs), or when all AEs were resolved.
Safety assessments
AEs were recorded from the time at which informed consent was granted until day 30 (±7) after treatment. AEs were defined as any unfavorable medical occurrence or worsening of subjective symptoms/objective findings, worsening of underlying diseases and complications, or clinically significant abnormal laboratory values. AEs were classified into mild, moderate, and severe according to severity. Serious AEs (SAEs) are defined as the following: (1) death or a life-threatening medical event; (2) hospitalization or the need to extend the hospitalization period; (3) continuous or meaningful disability or deterioration of function; (4) birth defects or abnormalities; or (5) other medically important situations. AEs were coded according to MedDRA, ver. 21.1 (ICH, Geneva, Switzerland).
Statistical analysis
Efficacy evaluation was done on the full analysis set (FAS) and per protocol set, with FAS being the main analysis group. Demographic and other baseline data were targeted at FAS, and safety data were evaluated using the safety set. No interim analysis or subgroup analysis was conducted. As descriptive statistics, the number of subjects, mean, standard deviation, median, minimum, and maximum were provided for continuous data; for categorical data, the number and percentage of subjects, and if necessary, frequency, were provided. For continuous data, the statistical significance of the difference between groups was tested with an independent 2-sample t-test if normality was satisfied, and a Wilcoxon rank sum test if normality was not satisfied. For categorical data, the statistical significance of the difference between groups was assessed with the Chi-square or Fisher’s exact test. For continuous data, the statistical significance of a within-group change was assessed with the paired t-test if normality was satisfied, and the Wilcoxon rank sum test if normality was not satisfied. For categorical data, the statistical significance of a within-group change was tested with McNemar’s test or McNemar’s exact test. All statistical analyses excluding the noninferiority assessment used a significance level of 0.05, and all statistical analyses were performed using SAS ver. 9.3 (SAS Institute).
RESULTS
Patient selection
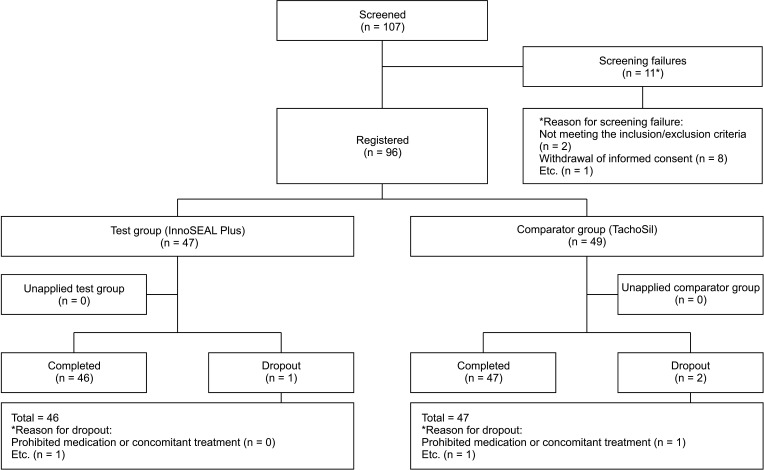
The study subjects were selected and divided into groups as illustrated in Fig. 1. Among 107 patients who were willing to participate in this study and give informed consent, 11 were not able to participate. Thus, a total of 96 patients were enrolled in the study. Eleven patients failed to pass screening for the following reasons; 2 patients did not meet the inclusion/exclusion criteria, 8 patients withdrew informed consent, and 1 patient canceled their planned liver transplantation surgery. In addition, there were 3 patients who dropped out after their surgical procedures because they did not complete their follow-up visits (1 for InnoSEAL Plus and 2 for TachoSil). Finally, 46 subjects in the test group and 47 subjects in the comparator group completed the trial (total of 93 patients).
Fig. 1. Flowchart of patient selection. Test group, patients treated by InnoSEAL Plus (InnoTherapy, Seoul, Korea); Comparator group, patients treated by TachoSil (Takeda Austria GmbH, Wien, Austria).
Patient characteristics
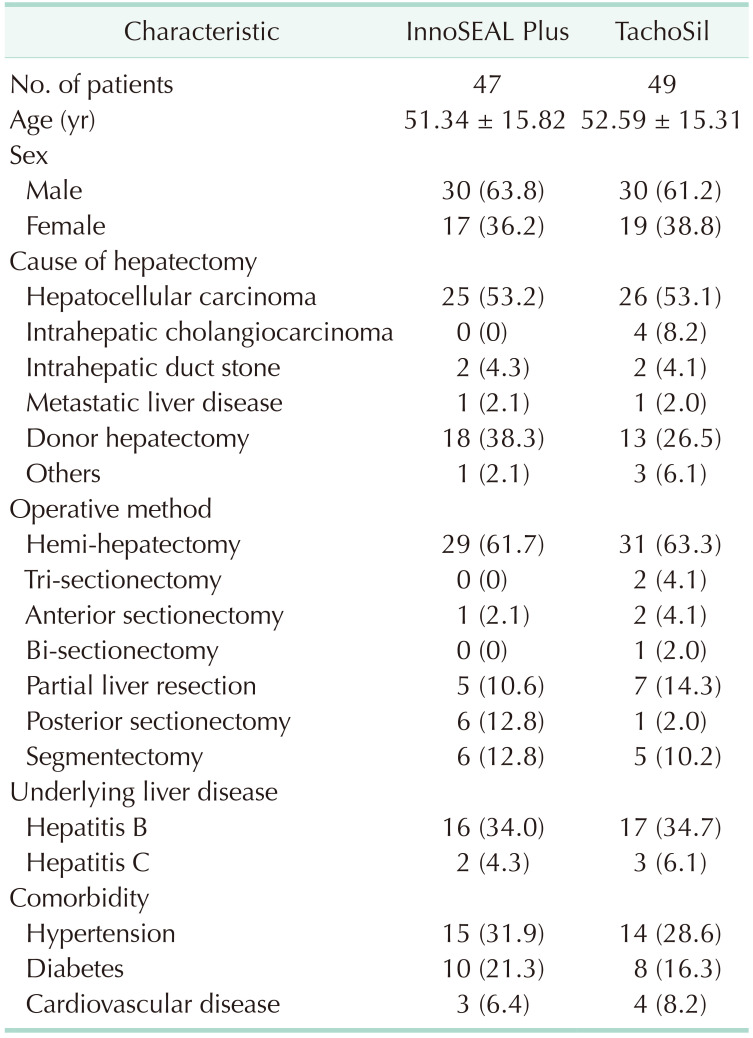
The average age of the study subjects was 51.34 ± 15.82 years in the InnoSEAL Plus group and 52.59 ± 15.31 years in the TachoSil group, and the overall average age was 51.98 ± 15.49 years. The sex ratios (male/female) were 63.8%:36.2% for InnoSEAL Plus and 61.2%:38.8% for TachoSil. Thus, overall, the sex ratio in the trial was 62.5% male and 37.5% female. Hepatocellular carcinoma (53.1%) and donor hepatectomy (32.3%) were the most common reasons for liver resection. Hemi-hepatectomy was the most common approach to liver resection. The rates of comorbidities were similar between the 2 groups. The baseline patient characteristics are summarized in Table 1.
Table 1. Baseline demographic characteristics of the study subjects.
Values are presented as number only, mean ± standard deviation, or number (%).
InnoSEAL Plus: InnoTherapy, Seoul, Korea; TachoSil: Takeda Austria GmbH, Wien, Austria.
Hemostatic efficacy analysis
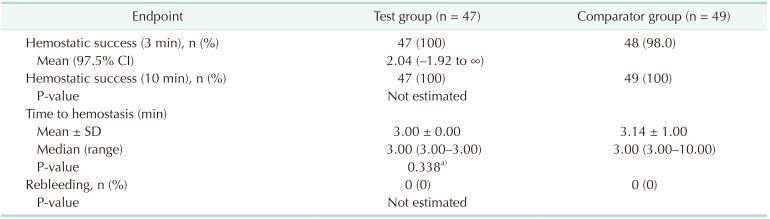
The hemostatic success rate was similar between the InnoSEAL Plus and TachoSil groups. The success rate of 3-minute hemostasis was 100% in the InnoSEAL Plus group (47 out of 47 patients). In the TachoSil group, the 3-minute success rate was 98.0% (48 out of 49 patients). The primary hemostatic outcome was thus nearly the same between the test and the comparator groups (Table 2). The difference in 3-minute success rate between the test and comparator groups was +2.04%. The calculated low side value for the 97.5% confidential interval was −1.92%. This value, −1.92%, was much higher than the statistical low limit inferiority margin, −23.9%. Therefore, the results of the clinical trial demonstrate that the hemostatic efficacy of InnoSEAL Plus is not inferior to that of TachoSil. The 10-minute hemostatic success rate was the same for both materials, at 100% efficacy. The average time required for successful hemostasis was 3.0 minutes for InnoSEAL Plus and 3.14 minutes for TachoSil, but the difference in time was not statistically meaningful. Rebleeding was not observed in either group.
Table 2. Hemostatic success rate (full analysis set).
Test group, patients treated by InnoSEAL Plus (InnoTherapy, Seoul, Korea); Comparator group, patients treated by TachoSil (Takeda Austria GmbH, Wien, Austria).
Full analysis set: all subjects who were randomly assigned to this clinical trial, treated with a test material or a comparator product, and evaluated for the primary efficacy endpoint.
a)Wilcoxon rank sum test.
Safety of hemostasis materials
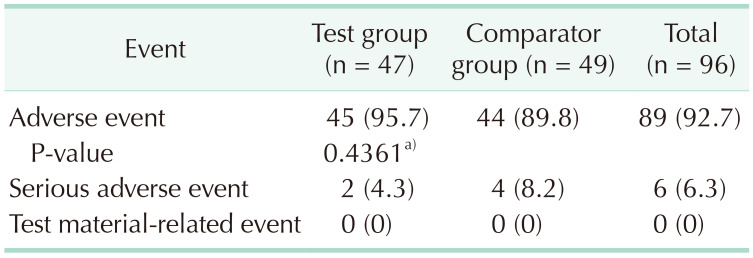
A total of 395 AEs (208 cases in the test group and 187 cases in the comparator group) were reported between the day of surgery and the end of the clinical trial. The total number of subjects who experienced more than 1 event was 89 (total, 92.7%; test group, 45 [95.7%]; comparator, 44 [89.8%]), but the difference between the groups was not statistically significant. SAEs were reported in 6 patients in total (6.3%): 2 patients in the test group (4.3%) and 4 in the comparator group (8.2%). There were no reports of abnormal cases that caused dropout (Table 3).
Table 3. Adverse events.
Values are presented as number of occurrences (%).
Test group, patients treated by InnoSEAL Plus (InnoTherapy, Seoul, Korea); Comparator group, patients treated by TachoSil (Takeda Austria GmbH, Wien, Austria).
a)Fisher’s exact test.
The SAEs that were observed in the test group were drug eruption and biloma. These conditions were not associated with the test materials. The SAEs that were observed in the comparator group were surgical site infection, abscess, pelvic inflammatory disease, and pneumonia. These were also deemed to be unrelated to the test materials.
DISCUSSION
The liver is a blood-rich organ, and there is a high risk of blood loss during hepatic surgery. Hepatic resection may result in continuous bleeding even after surgery because the resection surfaces remain exposed. For most other surgeries, the resection surfaces are sutured. Therefore, most studies on hemostatic agents have been conducted in patients undergoing hepatic resection [9,10,11,12]. Among hemostatic agents, physical polymeric agents such as cellulose, gelatin, starch, or collagen form a matrix at the site of bleeding which activates the extrinsic coagulation cascade and serves as a starting point for clot formation [13]. Thus, physical agents may not be appropriate in patients with severe coagulopathy because such agents require an intact coagulation cascade to function [14]. On the other hand, biologic hemostatic agents, such as topical thrombin and fibrin sealant, bypass the initial coagulation cascade to promote hemostasis through the common pathway [13]. Unlike physical polymeric agents, topical thrombin can be used in patients with impaired coagulation cascades, but adequate fibrinogen levels are required. Thus, such agents are useful in patients showing mild coagulopathy [15]. Therefore, TachoSil is the most widely used effective hemostatic agent in patients with liver resection, and most comparative studies have been conducted with TachoSil as the comparator [5,7]. For this reason, we designed a study to compare the novel compound with TachoSil for hemostasis in liver resection patients.
Patients with persistent oozing hemorrhage from transected liver surfaces after hepatectomy despite first-line hemostasis were enrolled in this study. To reduce bias, the surgeon did not know which product would be used on which patients before surgery. However, the different appearances of InnoSEAL Plus and TachoSil prevented us from performing a double-blinded clinical test.
This trial was designed to prove that the hemostatic efficacy of InnoSEAL Plus is not inferior to that of the comparator. The primary endpoint of this study was 3-minute hemostasis, and the secondary endpoint was 10-minute hemostasis. InnoSEAL Plus showed a 100% rate of 3-minute hemostasis (47 out of 47), while for TachoSil, the rate was 98.0% (48 out of 49 patients). The results of statistical analysis demonstrate that InnoSEAL Plus is not an inferior hemostatic material compared to TachoSil. Importantly, InnoSEAL Plus is a coagulation factor-free product, unlike TachoSil, which contains human thrombin and fibrinogen. Thus, the similar level of hemostatic efficacy demonstrated by InnoSEAL Plus is a surprising result. The mussel-inspired adhesive polymer, chitosan-catechol, in InnoSEAL Plus plays a key role in triggering immediate serum protein aggregation upon blood contact. Also, cellular components such as red blood cells and platelets are activated and triggered to aggregate by the hemostatic polymer. These complex biochemical adhesion reactions result in formation of sealing barriers at sites of hemorrhage [8]. The sealing barriers created by contact between the adhesive polymers in InnoSEAL Plus and serum proteins/blood cells are, notably, coagulation factor-free entities. Thus, the application of InnoSEAL Plus in coagulopathy patients remains to be explored in a follow-up clinical study.
As this is the first human study of a new hemostatic agent with entirely different hemostatic mechanisms, it is necessary to include a record of all AEs. Patients who were treated with InnoSEAL Plus after hepatectomy often showed mild gastrointestinal symptoms or other general disorders (~50%). These types of adverse effects are often observed in patients who undergo hepatectomy, and they were all successfully treated by medication. TachoSil was associated with a similar level of adverse symptoms after surgery. SAEs were 2 in the test group and 4 cases in TachoSil group. Among the 2 cases in the InnoSEAL Plus group, the first patient was diagnosed with a moderate fixed drug eruption and traumatic purpura which was treated with skin dressing and Advantan cream (LEO Pharma Manufacturing Italy S.r.l, Milano, Italy) until POD 18. The second patient showed biloma, so percutaneous abscess drainage (PCD) was performed. Gradual decreases in the amount of localized fluid from the hepatectomy beds were observed, and the patient was discharged on POD 22. Both SAEs were thought to have no direct relationship with the novel hemostatic material. The 4 cases in the TachoSil group were all related to bacterial infections. All were suitably treated and discharged (POD 31 for the 1st patient, POD 18 for the 2nd, POD 30 for the 3rd, and POD 26 for the 4th patient).
The limitations of this study are as follows. First, there was a minor difference in the standardized surgical procedures between surgeons in this multicenter study. Second, there may have been differences in the extent of bleeding depending on the resection methods and sites of resection used by different surgeons. Third, as only 96 patients participated in this clinical trial, we cannot definitively say that the rates of AEs will be similar between treatment groups in the general population. Further research is needed to address these issues.
In conclusion, this was the first clinical study to use the marine mussel-inspired catecholamine adhesive InnoSEAL Plus. The trial was designed to prove that the hemostatic efficacy of InnoSEAL Plus is not inferior to that of the comparator. The results demonstrate that InnoSEAL Plus is not an inferior hemostatic material compared to TachoSil. Considering that InnoSEAL Plus is a coagulation factor-free product, the similarities in hemostatic outcomes compared with TachoSil, which contains fibrinogen and thrombin, are very encouraging.
Footnotes
Fund/Grant Support: This study was supported by grants from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C0820).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization, Methodology: JHK.
- Formal Analysis: HL, JHK.
- Investigation: GSC, SHK, HIS, JHR, SPY.
- Project Administration: MYK, MSL, JHK.
- Writing — Original Draft: MYK, HL, JHK.
- Writing — Review & Editing: All authors.
References
- 1.Alfieri S, Carriero C, Caprino P, Di Giorgio A, Sgadari A, Crucitti F, et al. Avoiding early postoperative complications in liver surgery: a multivariate analysis of 254 patients consecutively observed. Dig Liver Dis. 2001;33:341–346. doi: 10.1016/s1590-8658(01)80089-x. [DOI] [PubMed] [Google Scholar]
- 2.Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. [DOI] [PubMed] [Google Scholar]
- 3.Kubo S, Takemura S, Yamamoto S, Hai S, Ichikawa T, Kodai S, et al. Risk factors for massive blood loss during liver resection for hepatocellular carcinoma in patients with cirrhosis. Hepatogastroenterology. 2007;54:830–833. [PubMed] [Google Scholar]
- 4.Spotnitz WD. Hemostats, sealants, and adhesives: a practical guide for the surgeon. Am Surg. 2012;78:1305–1321. [PubMed] [Google Scholar]
- 5.Ding H, Yuan JQ, Zhou JH, Zheng XY, Ye P, Mao C, et al. Systematic review and meta-analysis of application of fibrin sealant after liver resection. Curr Med Res Opin. 2013;29:387–394. doi: 10.1185/03007995.2013.768216. [DOI] [PubMed] [Google Scholar]
- 6.Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:426–430. doi: 10.1126/science.1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee H, Scherer NF, Messersmith PB. Single-molecule mechanics of mussel adhesion. Proc Natl Acad Sci U S A. 2006;103:12999–13003. doi: 10.1073/pnas.0605552103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim K, Ryu JH, Koh MY, Yun SP, Kim S, Park JP, et al. Coagulopathy-independent, bioinspired hemostatic materials: a full research story from preclinical models to a human clinical trial. Sci Adv. 2021;7:eabc9992. doi: 10.1126/sciadv.abc9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genyk Y, Kato T, Pomposelli JJ, Wright JK, Jr, Sher LS, Tetens V, et al. Fibrin sealant patch (TachoSil) vs oxidized regenerated cellulose patch (Surgicel Original) for the secondary treatment of local bleeding in patients undergoing hepatic resection: a randomized controlled trial. J Am Coll Surg. 2016;222:261–268. doi: 10.1016/j.jamcollsurg.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Fischer L, Seiler CM, Broelsch CE, de Hemptinne B, Klempnauer J, Mischinger HJ, et al. Hemostatic efficacy of TachoSil in liver resection compared with argon beam coagulator treatment: an open, randomized, prospective, multicenter, parallel-group trial. Surgery. 2011;149:48–55. doi: 10.1016/j.surg.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki S, Origasa H, Tetens V, Kobayashi M. Comparison of TachoSil and TachoComb in patients undergoing liver resection-a randomized, double-blind, non-inferiority trial. Langenbecks Arch Surg. 2017;402:591–598. doi: 10.1007/s00423-017-1571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C, Fu D, Wang F, Zhong X, Yang L, Wu G, et al. A randomized controlled trial to compare the efficacy of regenerated and non-regenerated oxidized cellulose gauze for the secondary treatment of local bleeding in patients undergoing hepatic resection. Ann Surg Treat Res. 2021;100:193–199. doi: 10.4174/astr.2021.100.4.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achneck HE, Sileshi B, Jamiolkowski RM, Albala DM, Shapiro ML, Lawson JH. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251:217–228. doi: 10.1097/SLA.0b013e3181c3bcca. [DOI] [PubMed] [Google Scholar]
- 14.Schreiber MA, Neveleff DJ. Achieving hemostasis with topical hemostats: making clinically and economically appropriate decisions in the surgical and trauma settings. AORN J. 2011;94:S1–S20. doi: 10.1016/j.aorn.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Shander A, Kaplan LJ, Harris MT, Gross I, Nagarsheth NP, Nemeth J, et al. Topical hemostatic therapy in surgery: bridging the knowledge and practice gap. J Am Coll Surg. 2014;219:570–579. doi: 10.1016/j.jamcollsurg.2014.03.061. [DOI] [PubMed] [Google Scholar]