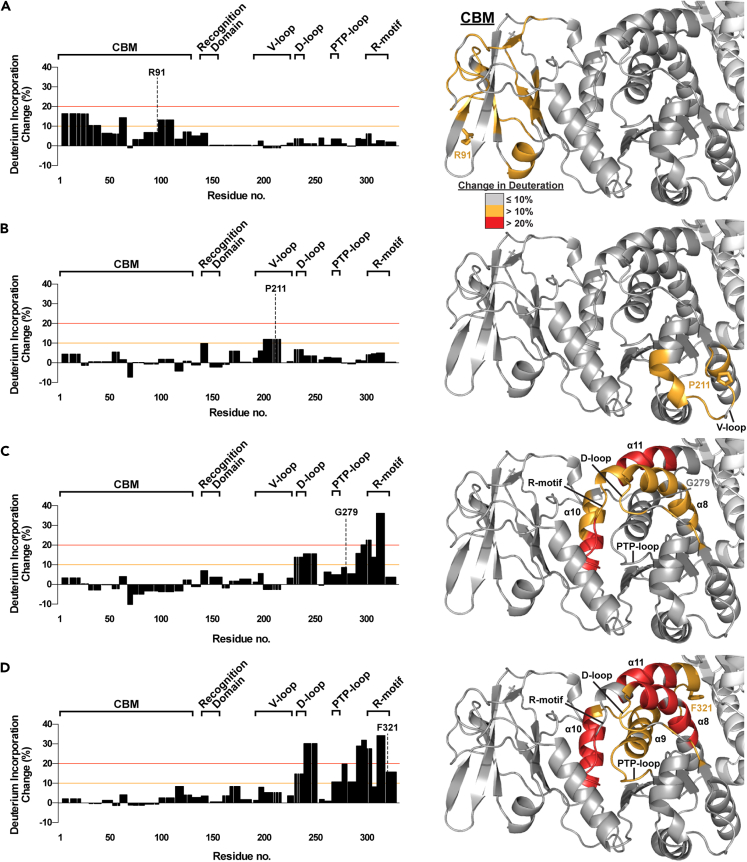
Figure 4.
HDX of select LD mutants
(A–D) (Left) Deuteration incorporation for each residue of the indicated mutant was determined from deuteration of overlapping peptides and compared with that of WT laforin. The optimized value for change in deuteration compared to WT laforin is plotted. Positive changes indicate an increase in solvent accessibility in the mutant; negative changes indicate a decrease in solvent accessibility in the mutant. The significance thresholds that were used for mapping significantly changed peptides onto the laforin crystal structure are marked by orange (10%) and red (20%) lines. The CBM and major motifs of the DSP domain, the recognition domain, V-loop, D-loop, PTP-loop, and R-motif, are indicated. (Right) Deuteration changes induced by the mutations are mapped onto one subunit of the laforin structure. Gray indicates a maximum change ≤10% from WT laforin, orange a change between 11 and 20% and red a change >20%. The side chain of the mutated residue is shown in stick representation and the residue is labeled. Structural features are labeled in black. Also see Figure S2, Datas S1, and S2.