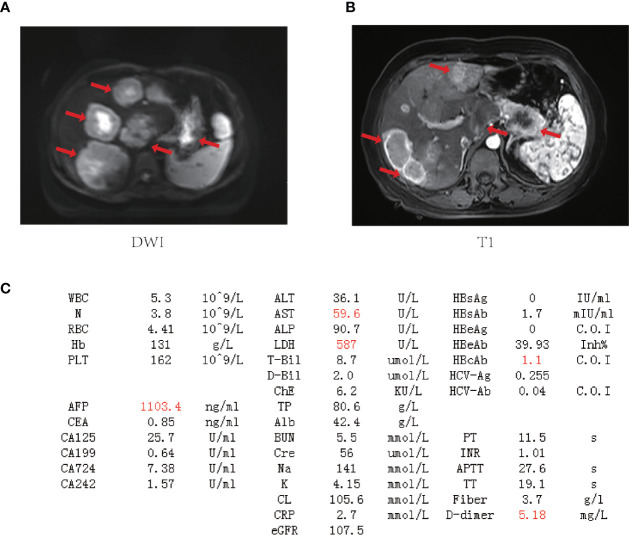
Figure 2.
The baseline laboratory and imaging examinations for the patient. (A, B) Magnetic resonance imaging (MRI) of the upper abdomen. Tumor lesions are marked by red arrows. (C) Laboratory data on admission (outliers are highlighted in red). WBC, white blood cell count; N, neutrophil count; RBC, red blood cell count; Hb, hemoglobin; PLT, blood platelet count; AFP, alpha-fetoprotein; CEA, carcinoembryonic antigen; CA125, carbohydrate antigen 125; CA199, carbohydrate antigen 19-9; CA724, carbohydrate antigen 724; CA242, carbohydrate antigen 242; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; T-Bil, total bilirubin; D-Bil, direct bilirubin; ChE, cholinesterase; TP, total protein; Alb, albumin; BUN, blood urea nitrogen; Cre, creatinine; Na, sodium; K, potassium; CL, chlorine; CRP, C-reactive protein; eGFR, glomerular filtration rate; HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface antibody; HBeAg, hepatitis B e antigen; HBeAb, hepatitis B e antibody; HBcAg, hepatitis B core antigen; HBcAb, hepatitis B core antibody; HCV-Ag, hepatitis C virus antigen; HCV-Ab, hepatitis C virus antibody; PT, prothrombin time; INR, international normalized ratio; APTT, activated partial thromboplastin time; TT, thrombin time.