Fig. 4.
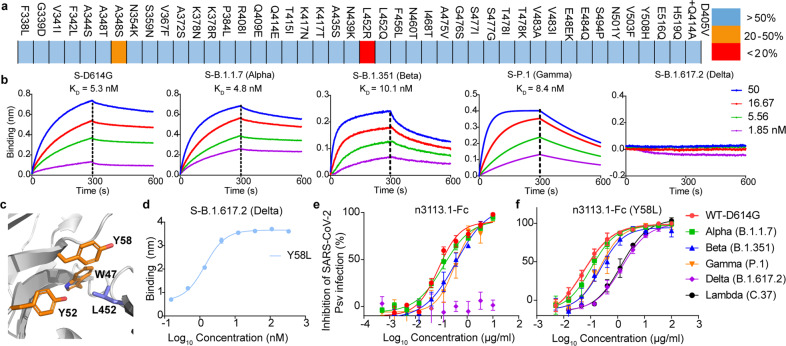
N3113.1 (Y58L) maintains susceptivity to circulating SARS-CoV-2 variants. a Binding affinity of n3113.1 with RBD variants. Colors represent the extent of binding affinity percentage relative to wild-type RBD: blue, over 50%; orange, 20% to 50%; red, less than 20%. b Binding kinetics of n3113.1-Fc to spike variants, as measured by BLI. KD values for n3113.1-Fc were obtained using a 1:2 bivalent model. c Binding mode of RBD-L452 and n3113. RBD and n3113 are shown as cartoon, colored in blue and orange, respectively. RBD-L452 and residues on n3113 that form hydrophobic interactions are shown as sticks. d Binding of Y58L mutations based on n3113.1-Fc to S protein of Delta variant. The mean ± SD from two independent experiments is shown. e Neutralization profiles for n3113.1-Fc against viruses pseudotyped with the S protein of WT and four VOCs. Two independent experiments were performed in triplicate. f Neutralization profile of n3113.1-Fc (Y58L) against viruses pseudotyped with the S protein of WT, four VOCs, and one VOI. The mean ± SD from three independent experiments is shown