Figure 3.
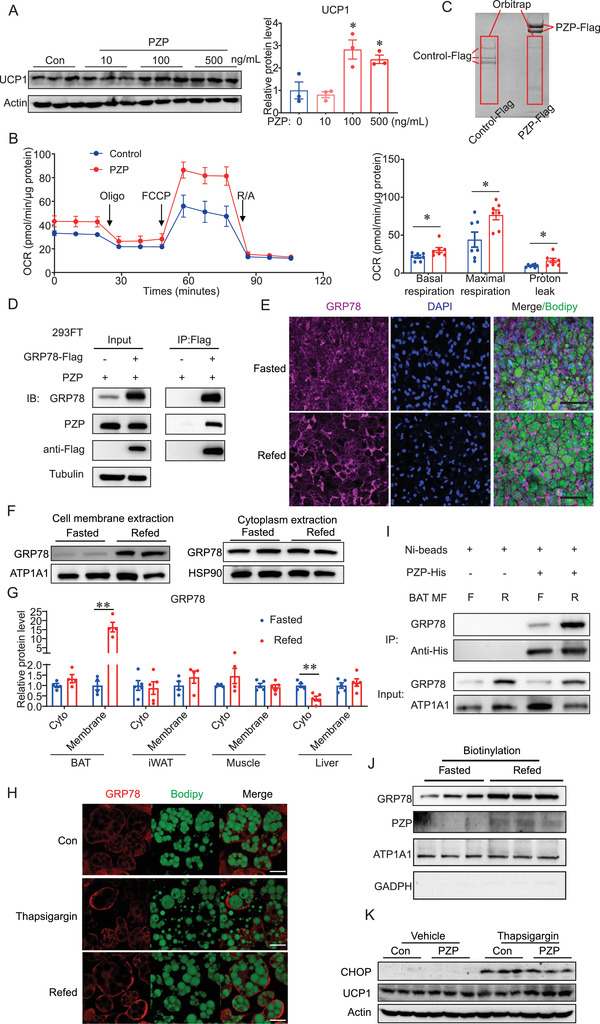
PZP promotes UCP1 expression through binding to cell surface GRP78 during refeeding. A) Immunoblots of cell lysate from differentiated BA which were cultured in refeeding medium supplemented with PZP protein (10, 100, 500 ng mL−1). B) Oxygen consumption of differentiated BA which were cultured in refeeding medium supplemented with PZP protein (100 ng mL−1) showed higher basal cellular respiration, maximal respiration, and proton leak than control group. C) SDS‐PAGE gel of anti‐Flag immunoprecipitation from 293FT cell lysate which co‐overexpressed BAT cDNA library and PZP‐Flag or Control‐Flag. Red square indicates the chopped gel which we sent for orbitrap analysis. D) 293FT was overexpressed with GRP78‐Flag and PZP. PZP‐GRP78 complex was immunoprecipitated by anti‐Flag antibody and blotted with indicated antibodies. E) Whole mount tissue staining images of iBAT from fasted and refed mice using anti‐GRP78 which showed GRP78 in BAT translocated from cytoplasm to cell membrane under IF. F) Immunoblots of cell membrane and cytoplasmic fractions from BAT using indicated antibodies which showed GRP78 dramatically increased in cell membrane fraction rather than in cytoplasm upon refeeding. G) Quantification of GRP78 protein level in cell membrane and cytoplasmic fractions of BAT, iWAT, muscle and liver from fasted and refed mice. H) Representative fluorescence images of BA which expressed GRP78‐cherry fusion protein after refeeding and thapsigargin treatment which showed refeeding and thapsigargin treatment induced translocation of GRP78 to cell surface of BA. I) Cell membrane extraction from BAT incubated with PZP‐His protein. PZP‐GRP78 complex was immunoprecipitated by anti‐His beads and blotted with indicated antibodies which showed refeeding increased GRP78‐PZP complex formation in the plasma membrane fraction of BAT. F, fasting; R, refeeding. Right panel shows result of quantitative analysis. J) Immunoblots of cell surface protein from differentiated BA which were cultured in fasting or refeeding medium supplemented with PZP protein (100 ng mL−1). Cell surface proteins were biotinylated and isolated by EZLabel Protein Biotin Labeling Kit and Streptavidin beads. K) Immunoblots of cell lysate from differentiated BA which were cultured supplemented with thapsigargin (600 µm) and PZP protein (100 ng mL−1) using indicated antibodies. Data are shown as mean ± SEM. One‐way (A) or two‐way ANOVA (B,G) with multiple comparisons and Tukey's post‐test were performed; *** p <0.001, ** p < 0.01, and * p < 0.05 were considered to be significant.
