Figure 4.
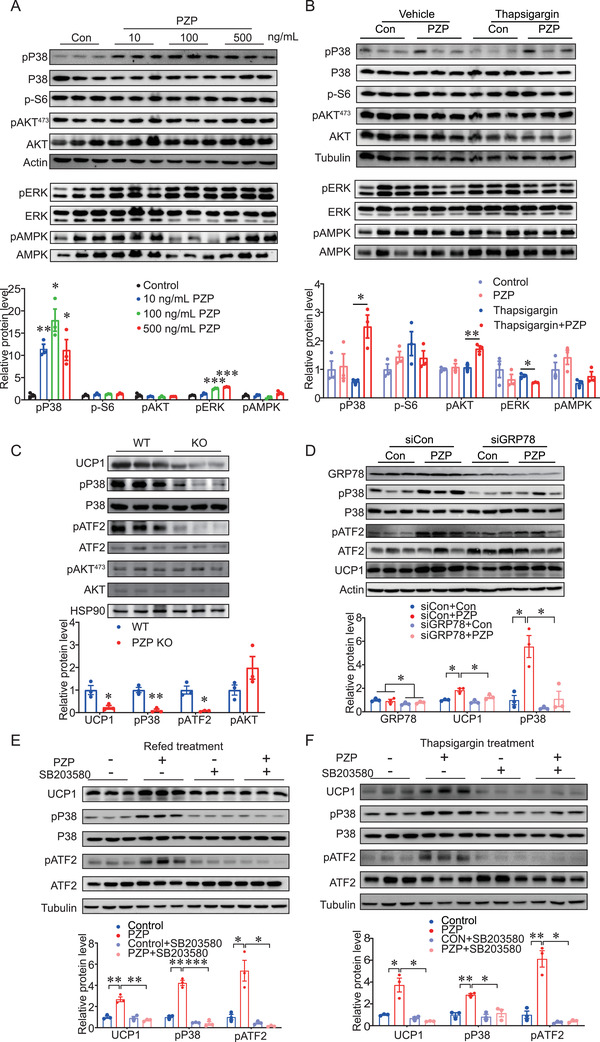
PZP promotes UCP1 expression via a P38 MAPK‐ATF2 pathway. A) Immunoblots of cell lysate from differentiated BA which were cultured in refeeding medium supplemented with PZP protein (10, 100, 500 ng mL−1) using indicated antibodies which showed PZP significantly increased the phosphorylation of p38 MAPK in condition of refeeding. B) Immunoblots of cell lysate from differentiated BA which were cultured supplemented with or without thapsgargin (600 µm) and PZP protein (100 ng mL−1) using indicated antibodies. C) Immunoblots of BAT lysates from control mice and PZP KO mice when mice were fed upon HFD and IF after 2 weeks without difference in body weight between groups using indicated antibodies which showed phosphorylation of p38 MAPK and ATF2 were decreased in PZP KO mice. The samples were collected when mice were refed for 6 h after 24‐h fast. D) Immunoblots of cell lysate from siGRP78‐transfected BA and controls which were cultured in refeeding medium supplemented with or without PZP protein (100 ng mL−1) using indicated antibodies, which showed UCP1 expression and phosphorylation of p38 MAPK and ATF2 upon PZP treatment were significantly inhibited by GRP78 siRNA. E) Immunoblots of cell lysate from differentiated BA which were cultured in refeeding medium supplemented with or without SB203580 (10 µm) and PZP protein (100 ng mL−1) using indicated antibodies, which showed PZP‐induced UCP1 expression under refeeding condition was significantly inhibited by SB203580 treatment. F) Immunoblots of cell lysate from differentiated BA which were cultured supplemented with or without SB203580 (10 µm) and PZP protein (100 ng mL−1) after thapsigargin (600 µm) stimulation using indicated antibodies, which showed PZP induced UCP1 expression under thapsigargin treatment was significantly inhibited by SB203580 treatment. Data are shown as mean ± SEM. One‐way (A–C) or two‐way ANOVA (D–F) with multiple comparisons and Tukey's post‐test were performed; *** p <0.001, ** p < 0.01, and * p < 0.05 were considered to be significant.
