Figure 5.
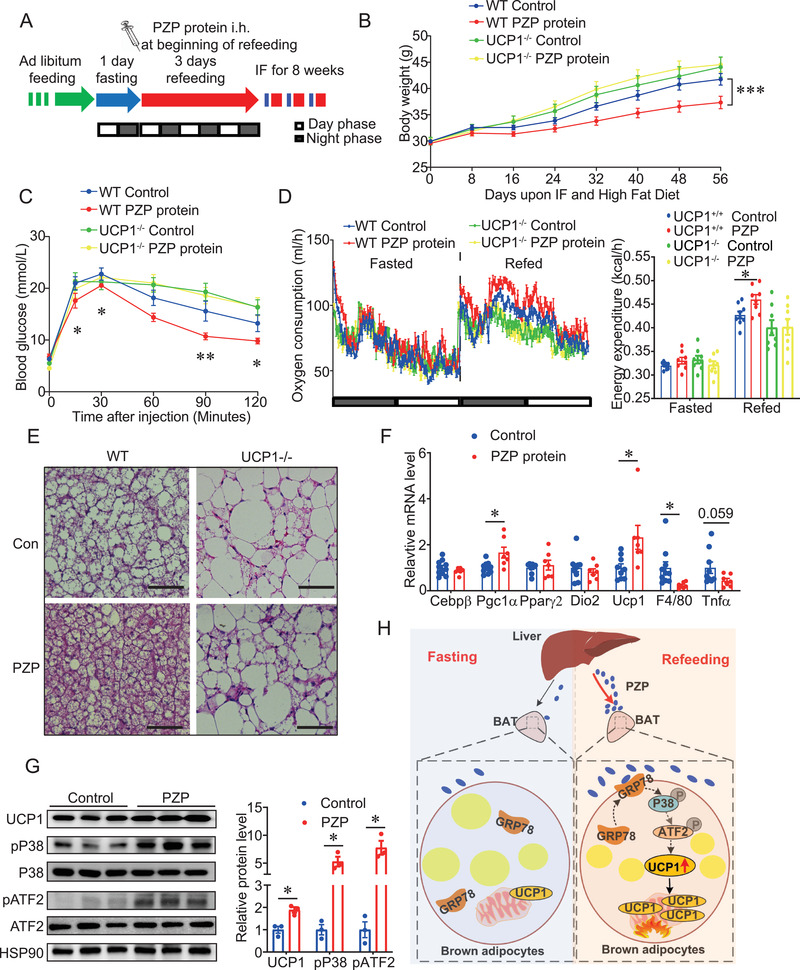
Increased circulating PZP augments UCP1‐dependent energy expenditure and protects DIO during IF treatment WT and UCP1−/− mice chronically subjected to HFD for 7 weeks. A) Schematic shows the procedure of in vivo therapeutic experiments. Mice were fed in IF regimen, comprising 1 day of fasting followed by 3 days of feeding per cycle for 8 weeks. We performed i.h. of PZP protein at the beginning of refeeding. Blue color indicating fasting; red color indicating refeeding. B) Body weight and C) GTT of WT and UCP1−/− mice treated with or without PZP protein (1 mg/kg) are shown (n = 8–10). D) Oxygen consumption rate (left panel) and energy expenditure (right panel) of WT and UCP1−/− mice treated with or without PZP protein (n = 8–9). These experiments were performed when mice were upon HFD and IF after 2 weeks without difference in body weight between groups, which showed PZP protein injection dramatically increased the oxygen consumption and energy expenditure of WT mice but not UCP1−/− mice. E) Representative H&E staining images of iBAT from WT and UCP1−/− mice treated with or without PZP protein (scale bar = 100 µm). F) Relative mRNA expression of adipogenic, thermogenic, and inflammatory genes in BAT from WT mice treated with or without PZP protein (n = 7–10). G) Immunoblots of total BAT lysates from WT mice treated with or without PZP protein using indicated antibodies which showed PZP protein treatment activated p38 MAPK‐ATF2‐UCP1 signaling in WT mice (n = 3). H) Schematic overview of main findings. Refeeding signals induced liver to produce and release PZP into circulation and followed by the translocation of GRP78 to cell surface in BAT. Subsequently, circulating PZP could bind to cell surface GRP78 to promote UCP1 expression via a p38 MAPK‐ATF2 signaling pathway in BAT. Data are shown as mean ± SEM. Two‐tailed Student's t‐test (F,G) or two‐way repeated measures ANOVA (B–D) with multiple comparisons and Tukey's post‐test were performed; ***p<0.001, ** p < 0.01, and * p < 0.05 were considered to be significant.
