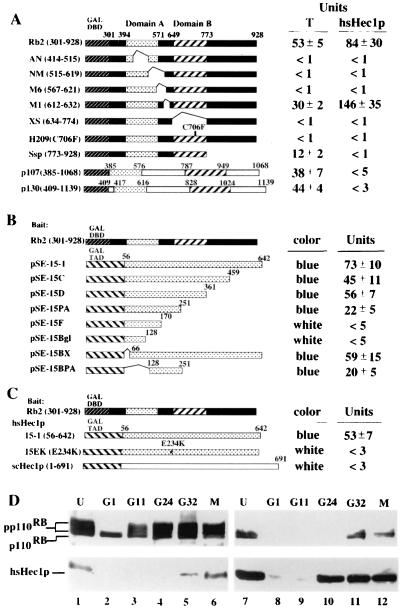
FIG. 1.
Specific interaction between Rb and hsHec1p. (A) hsHec1p and T antigen bind to similar regions of the Rb protein. The Gal4 DNA-binding domain (DBD) (amino acids 1 to 147; stippled box) was fused to various Rb mutants, p107 (amino acids 385 to 1068), or p130 (amino acids 409 to 1139). The simian virus 40 T-antigen-binding domains A and B are shown as shaded and hatched boxes, respectively. hsHec1p or T antigen (13) was expressed as a Gal4 transactivation domain fusion protein and used to test for interaction with Rb fusion proteins in yeast two-hybrid assays. Transformants were grown in liquid cultures and used for o-nitrophenyl-β-d-galactopyranoside quantitation of β-galactosidase activity as described previously (13). (B) Various hsHec1p mutants were fused with the Gal4 transactivation domain (TAD) (hatched box). Rb (amino acids 301 to 928) was expressed as the fusion with the Gal4 DNA-binding domain used for panel A. (C) Rb was expressed as the same fusion used for panel B. Wild-type hsHec1p (15-1), an hsHec1p mutant with amino acid 234 changed from E to K (15EK), and scHec1p were fused with the Gal4 transactivation domain. (D) Cell cycle-dependent interaction between Rb and hsHec1p. T24 cells were density arrested at G1 (lanes 2 and 8) and then released for reentry into the cell cycle. At different time points after release as indicated above the lanes, 5 × 106 cells were collected, lysed, and immunoprecipitated with anti-Rb MAb 11D7 (lanes 1 to 6) or with anti-hsHec1p MAb 9G3 (lanes 7 to 12). The immune complexes were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by immunoblotting with MAb 11D7 (upper panel) or with 9G3 (lower panel). G11 represents 11 h after release and corresponds to G1, G24 marks 24 h after release and corresponds to S, and G32 marks 32 h after release and corresponds to G2. M phase lysates (lanes 6 and 12) were obtained from cells treated with nocodazole (0.4 μg/ml). Lanes 1 and 7, unsynchronized cells.