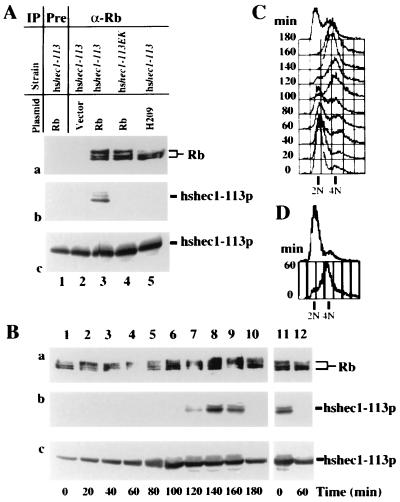
FIG. 2.
Reconstitution of the interaction between Rb and hsHec1p in yeast. (A) Specific interaction between hsHec1p and Rb. Yeast cells were diluted to an optical density at 600 nm of 0.75 in fresh medium with 2% galactose and 2% raffinose and then cultured at 30°C for 4 h. Aliquots of cell lysate were immunoprecipitated (IP) with nonspecific IgG (lane 1) or with anti-Rb (α-Rb) MAb 11D7 (lanes 2 to 5) and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The same blot was probed with MAb 11D7 for Rb (a) or MAb 9G3 for hshec1-113p (b). Panel c shows the endogenous level of hshec1-113p in the cells used in panels a and b. For each lane in panel c, aliquots of the same lysates used in panel a were immunoprecipitated and immunoblotted with 9G3. The yeast strains and plasmids used to express Rb are indicated for each lane. (B) The yeast cells carrying the hshec1-113 allele and an Rb expression vector were treated for 5 h with 0.1 M hydroxyurea or 20 μg of nocodazole per ml in medium containing 2% galactose and 2% raffinose. At different time points after release (indicated under each lane [lanes 1 to 10, release from hydroxyurea; lanes 11 and 12, release from nocodazole]), cells were collected, lysed, and immunoprecipitated with α-Rb MAb 11D7. The immunoprecipitates were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by immunoblotting with 11D7 for Rb (a) or with 9G3 for hshec1-113p (b). Aliquots of the same lysates were immunoprecipitated and immunoblotted with 9G3 (c). hshec1-113p was co-precipitated by Rb specifically at 120 to 160 min after release from hydroxyurea treatment, corresponding to G2/M phase, or metaphase arrest by nocodazole (time zero, lane 11). (C and D) The DNA content of the same cells used for panel B was analyzed by fluorescence-activated cell sorting as described previously (59).