Figure 3. Noninferiority Sensitivity and Subgroup Analyses for the Primary End Point for the Amoxicillin Dose and Dose Duration Randomizations.
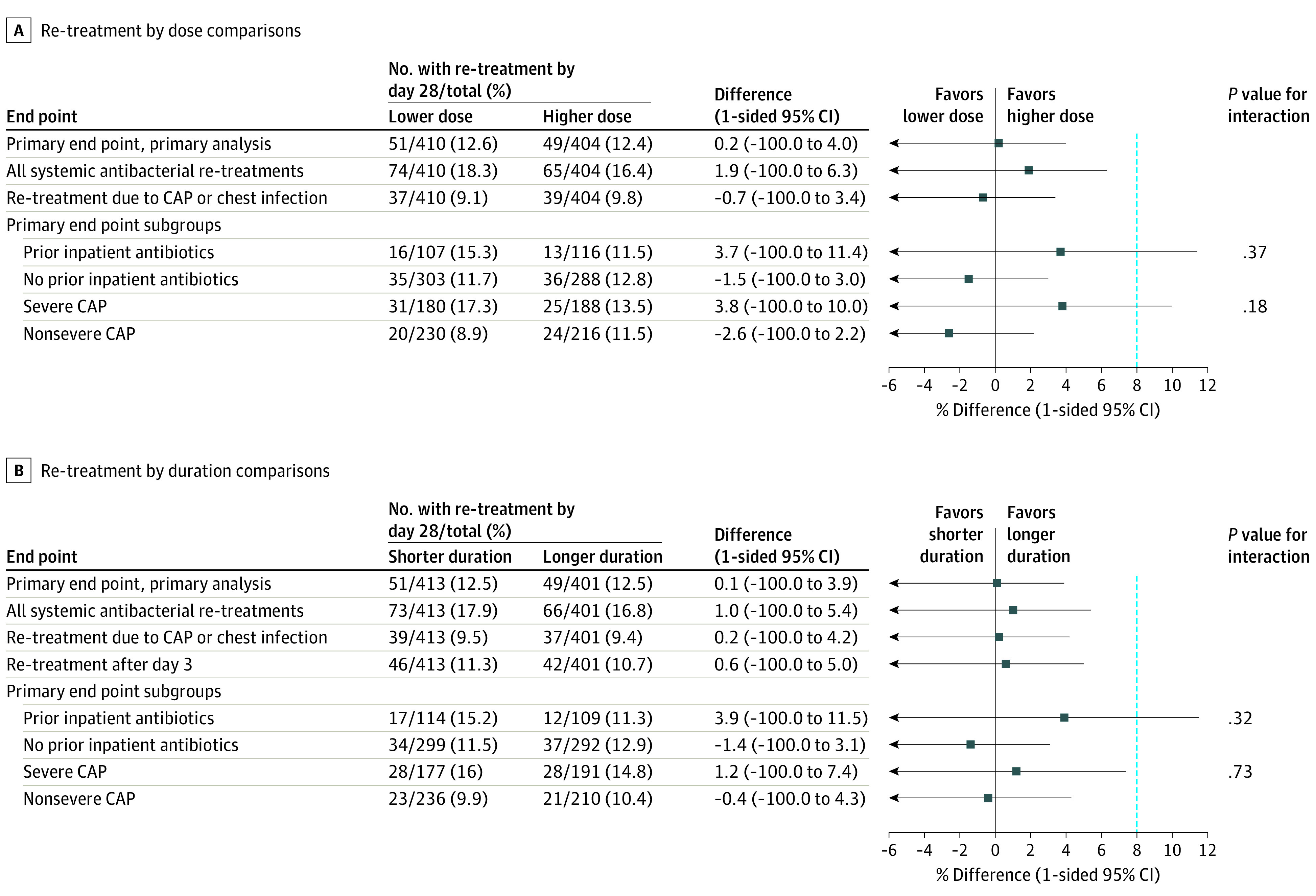
The primary analysis and 3 prespecified analyses are shown for both randomizations including all systemic antibacterial re-treatments, only re-treatments for community-acquired pneumonia (CAP) or chest infection, and by severe CAP subgroups. In addition, a post hoc subgroup analysis by prior inpatient antibiotic exposure is shown. A sensitivity analysis including only re-treatments after day 3 is shown for the duration randomization. One-sided 95% CIs are shown with the lower bound extending to −100%. The blue dashed vertical line at 8% indicates the noninferiority margin.
