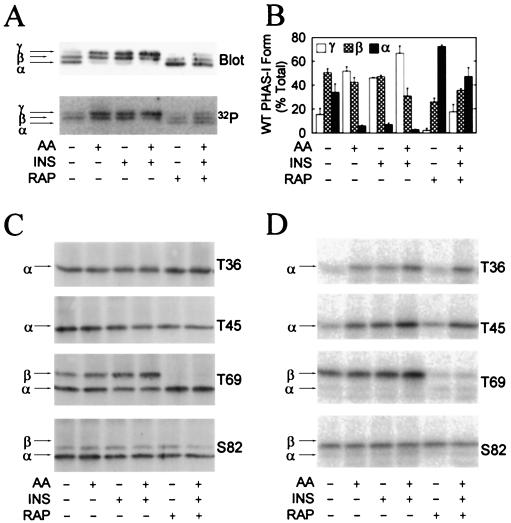
FIG. 4.
Stimulation of PHAS-I phosphorylation by amino acids and insulin. Cells expressing WT PHAS-I, T36 PHAS-I, T45 PHAS-I, T69 PHAS-I, or S82 PHAS-I were incubated with 32Pi (see protocol 1 in Materials and Methods) for 3 h before extracts were prepared. Prior to terminating the incubations, the cells were treated as follows: no additions, amino acids [AA] and/or insulin [INS] for 30 min, rapamycin [RAP] for 90 min, and rapamycin for 60 min followed by rapamycin plus insulin and amino acids for 30 min. PHAS-I proteins were immunoprecipitated from extracts, subjected to SDS-PAGE, and transferred to Immobilon membranes. (A) Immunoblot depicting the α, β, and γ forms of WT PHAS-I (Blot) and a phosphorimage showing the 32P-labeled protein (32P). (B) Relative proportions of α, β, and γ of WT PHAS-I. The optical density of each of the forms was determined by scanning laser densitometry and is expressed as a percentage of the total. Means and standard errors from three experiments are presented. (C) Immunoblots showing T36 PHAS-I, T45 PHAS-I, T69 PHAS-I, and S82 PHAS-I. (D) Phosphorimages showing the 32P-labeled mutant proteins.