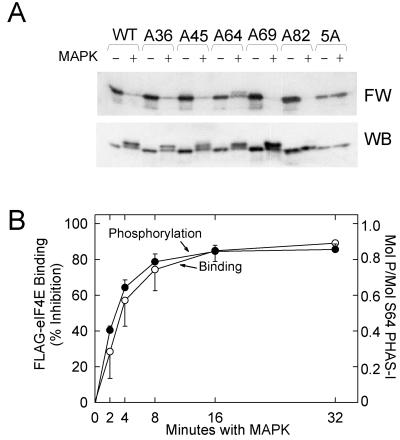
FIG. 6.
Effect of phosphorylating mutant PHAS-I proteins in vitro on eIF4E binding. (A) Proteins were incubated with ATP in the absence (−) or presence (+) of MAP kinase (MAPK) for 5 h. Samples were subjected to SDS-PAGE before the proteins were transferred to an Immobilon membrane. A far Western blot (FW) prepared using 32P-labeled FLAG-eIF4E and a PHAS-I immunoblot are shown. (B) S64 PHAS-I was incubated for increasing times with recombinant MAP kinase and either [γ-32P]ATP or unlabeled ATP before samples were subjected to SDS-PAGE. Gels containing 32P-labeled samples were stained with Coomassie blue. The S64 PHAS-I bands were excised, and the 32P contents were determined by scintillation counting. The results are expressed as moles of phosphate incorporated per mole of S64 PHAS-I. S64 PHAS-I that had been phosphorylated with unlabeled ATP was transferred to an Immobilon membrane, which was probed with 32P-labeled FLAG-eIF4E. The results represent the inhibition of binding due to MAP kinase. The results for phosphorylation (●) and FLAG-eIF4E binding (○) are mean values and standard errors from three experiments.