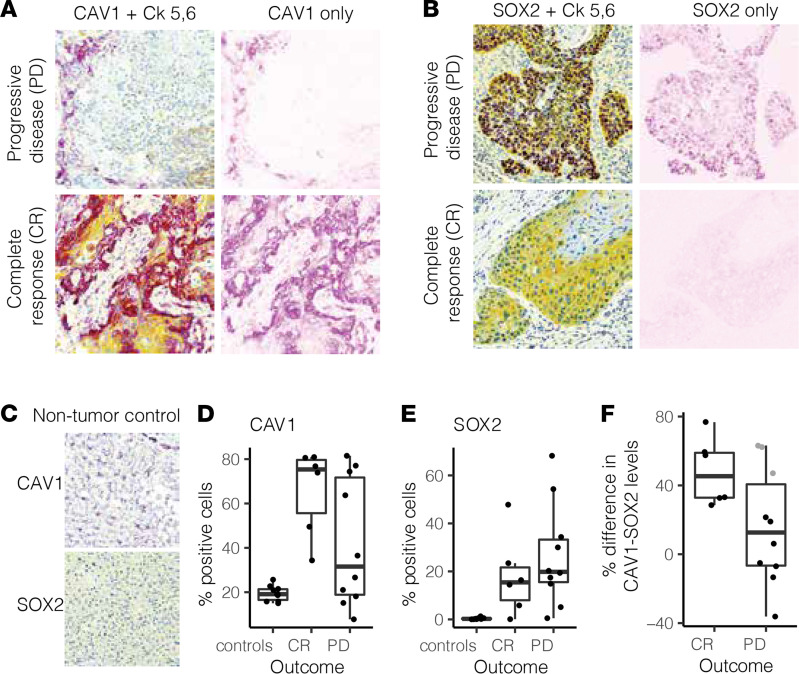
Figure 6. Validation of caveolin-1 and Sox-2 as biomarkers in HNSCC clinical samples from patients with known clinical responses to cetuximab.
(A) Representative images of tumor sections from patients with progressive disease (PD; top) or complete response (CR; bottom), proxies for resistance and sensitivity, respectively. Cells are stained for caveolin-1 (purple), cytokeratin 5,6 (yellow), and DNA (blue). Image deconvolution was applied computationally to extract the caveolin-1–only part of the image. (B) Representative images of tumor sections from patients with progressive disease (top) or complete response (bottom), proxies for resistance and sensitivity, respectively. Cells were stained for Sox-2 (purple), keratin (yellow), and DNA (blue). Image deconvolution was applied computationally to extract the Sox-2–only part of the image. (C) CAV1 (top) and SOX2 (bottom) staining in nontumor tissue control samples (healthy human hepatocytes). (D) The percentage of cells positive for caveolin-1 staining was calculated for each tumor section and segregated by grouping: complete response, progressive disease, or controls (healthy human hepatocytes). (E) The percentage of cells positive for Sox-2 staining was calculated for each tumor section and segregated by grouping: complete response, progressive disease, or controls. (F) The within-tumor section difference between percentages of caveolin-1–positive and Sox-2–positive cells for patients experiencing complete response or progressive disease in response to cetuximab treatment. Gray dots denote tumor sections that may be misclassified according to this metric. When available, multiple tumor sections are included from the same patient (total tumor sections = 16, total patients = 9). Original magnification, ×20 (A–C).