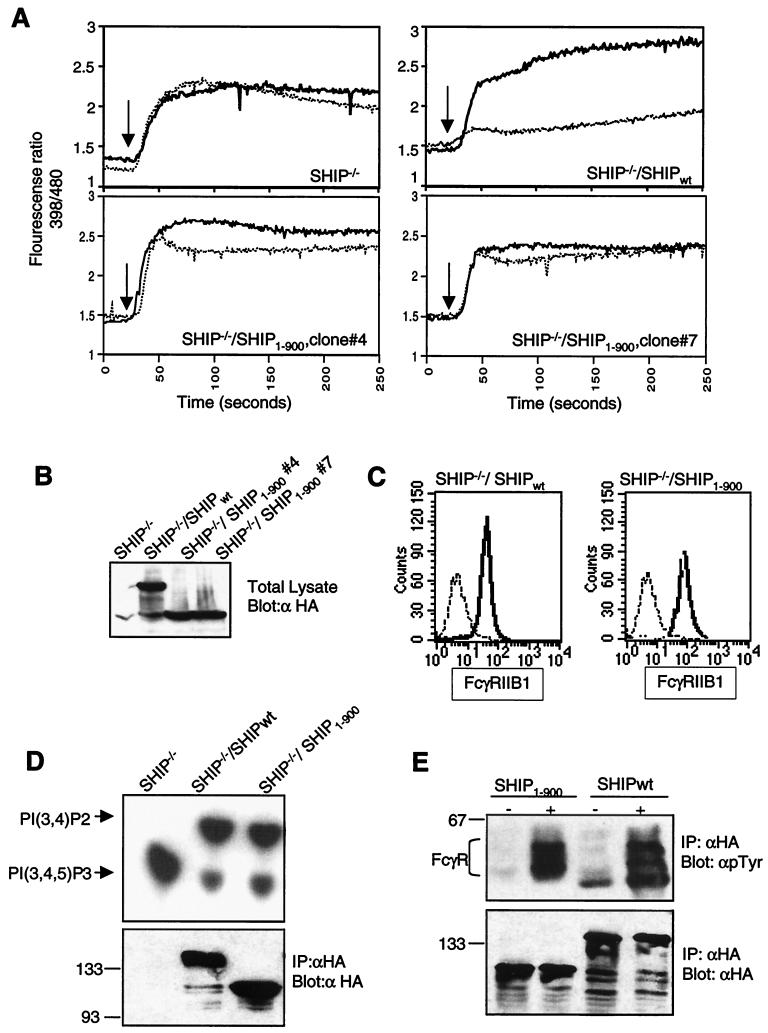
FIG. 3.
C terminus of SHIP required for inhibition of calcium flux by FcγRIIB1. (A) SHIP−/− DT40 cells and transfectants expressing HA-tagged wt SHIP or SHIP truncated at amino acid 900 (SHIP1–900) were analyzed for calcium flux upon BCR cross-linking (heavy solid line) or BCR plus FcR co-cross-linking (dotted line) as described in the legend to Fig. 2. (B) Expression of SHIPwt and SHIP1–900 in the DT40 clones used for calcium analysis in panel A was analyzed by immunoblotting. (C) Expression of FcγRIIB1 on DT40 transfectants used in the above experiments was analyzed by flow cytometry. FcR-negative parental DT40 cells were used as a negative control (dashed line). The SHIPwt transfectant (left panel) and the two SHIP1–900 clones (right panel) are shown as solid lines. (D) Phosphatase activity of SHIPwt and SHIP1–900 proteins immunoprecipitated (IP) from DT40 clones with anti-HA antibody analyzed with PIP3 as the substrate. The lower panel shows the immunoblot of precipitated proteins with anti-HA antibody. Sizes are shown in kilodaltons. (E) Coprecipitation of phosphorylated FcγRIIB1 with SHIPwt and SHIP1–900 proteins in DT40 clones. Cells were either left unstimulated or activated by coligation with BCR plus FcR for 5 min and lysed, and SHIP proteins were immunoprecipitated with anti-HA antibody and analyzed by immunoblotting with antiphosphotyrosine (γpTyr) antibody (upper panel) or anti-HA antibody (lower panel).