Figure 5.
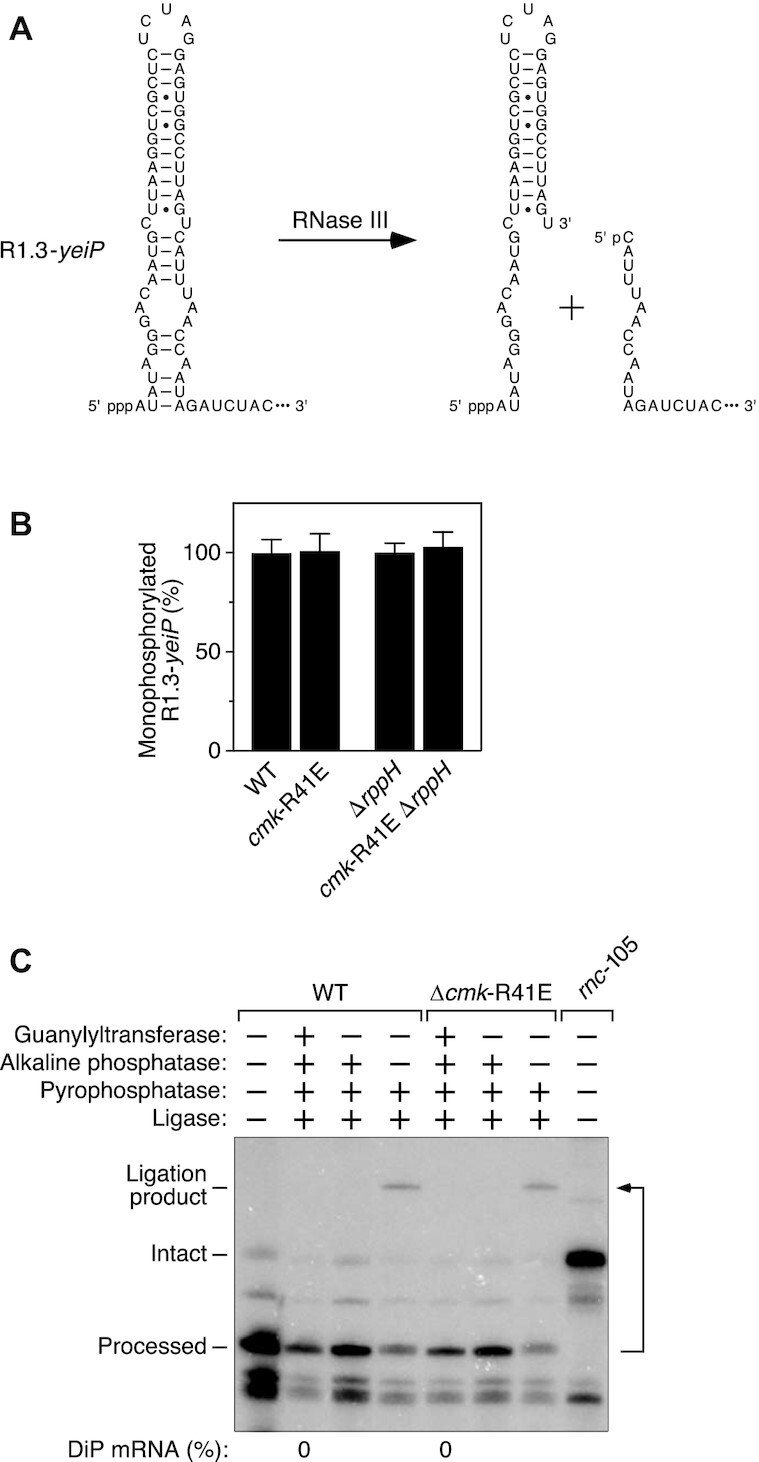
Inability of cytidylate kinase to phosphorylate a C-initiated E. coli RNA bearing a 5′ monophosphate. (A) RNase III cleavage within the 5′ untranslated region of R1.3-yeiP mRNA to generate a processed product that is monophosphorylated and begins with C. Base pairs are indicated by horizontal lines. The remainder of each RNA (not shown) is represented by an ellipsis. The natural 5′ untranslated region of wild-type yeiP mRNA begins eight nucleotides (ACACACAC) downstream of the sequence shown. p, phosphate. (B) Invariant percentage of processed R1.3-yeiP 5′ ends that are monophosphorylated in cells lacking cytidylate kinase activity. The phosphorylation state of the processed 5′ end of R1.3-yeiP RNA in an isogenic set of E. coli strains was analyzed by PABLO, as in Figure 1. Each value is the average of three biological replicates. Error bars correspond to standard deviations. (C) Absence of processed R1.3-yeiP 5′ ends that are diphosphorylated. The phosphorylation state of the processed 5′ end of R1.3-yeiP RNA in an isogenic pair of E. coli strains containing or lacking cytidylate kinase activity was analyzed by PACO, as in Figure 3. No processed 5′ ends capable of being capped by the guanylyltransferase Pce1 were detected in either strain, as evidenced by the absence of a splinted ligation product after consecutive treatment with all four enzymes (DiP mRNA: 0%). As expected, the R1.3-yeiP transcript did not undergo processing by RNase III in a mutant strain (rnc-105) lacking that endonuclease. A representative experiment is shown.
