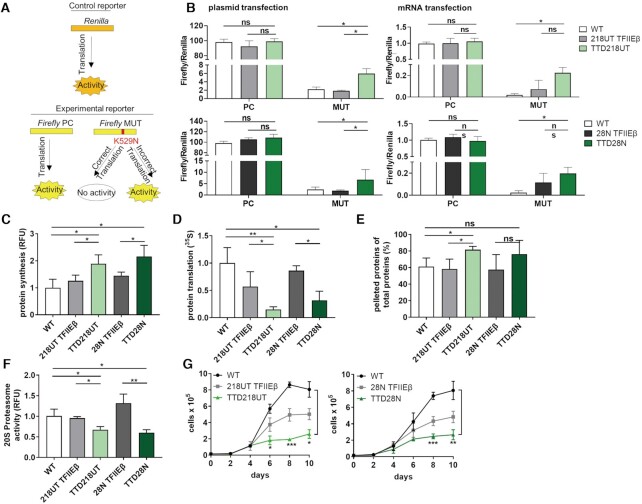
Figure 5.
Loss of protein homeostasis in TTD cells. (A) Luciferase-based translation fidelity assay includes a control reporter Renilla and two Firefly reporters containing either no mutation (PC) or a point mutation K529N inactivating the Firefly (MUT). With correct translation, the point mutation is translated and Firefly is inactive, whereas with inaccurate translation the luminescence of the active Firefly is detected. The Firefly luminescence was normalized to the Renilla luminescence. (B) Left: To all cells, plasmids coding for Renilla were co-transfected via electroporation with either plasmids coding for Firefly NC or Firefly MUT. Translation fidelity assay with plasmids shows increased translational infidelity in TTD cells. Right: Plasmids coding for Renilla/Firefly PC or Renilla/Firefly MUT were transcribed by T7 polymerase and resulting mRNAs were transfected with lipofectamine to all cells. Translation fidelity assay with mRNAs indicates increased translational infidelity in TTD cells. (C) 5 FAM-Azide detection of OPP-labeled proteins indicates the rate of protein synthesis initiation in WT, reconstituted and TTD cells. TTD cells show increased translation initiation. (D) Analysis of total protein translation by using 35S metabolic labeling shows decreased protein synthesis in TTD cells compared to WT and reconstituted cells. (E) Heat sensitivity analysis of cytoplasmic extract in WT, reconstituted and TTD cells show increased percentage of pelleted proteins in TTD cells after heat treatment for 15 min at 99°C. (F) Detection of cleaved 20S specific substrate, SUC-LLVY-AMC, indicating the 20S proteasome activity in WT, reconstituted and TTD cells. Reduced proteasome activity was observed in TTD cells. (G) Proliferation kinetics of TTD cells compared to WT and reconstituted cells indicate reduced cell proliferation in TTD cells. Data are represented as mean ± SD of at least three independent experiments. ns P > 0.05, * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001.