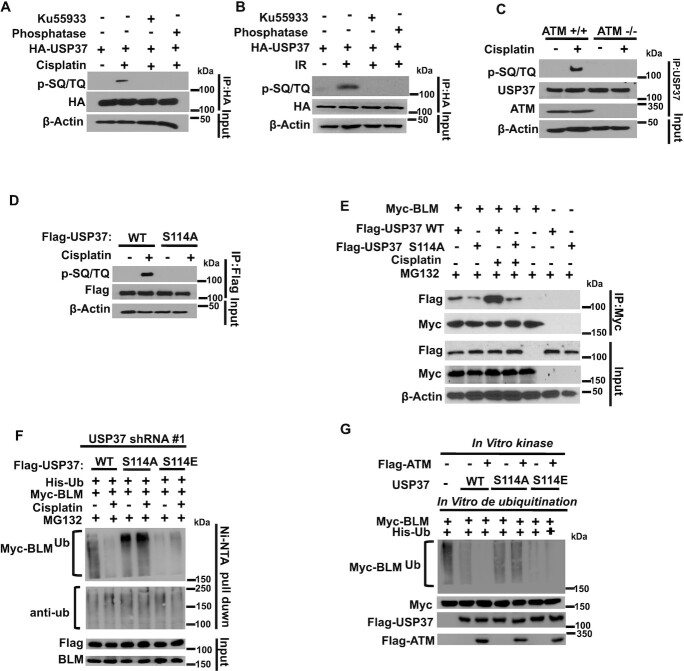
Figure 5.
Regulation of USP37 by DDR signaling. (A) HEK293T cells stably expressing HA-USP37were pretreated with DMSO or 10 μM Ku55933 for 4 h followed by mock treatment or cisplatin (3 μmol/l) for 16 h. After that, HA-USP37 was immunoprecipitated, left untreated or treated with phosphatase, and immunoblotted with phospho-SQ/TQ (pSQ/TQ) antibody. (B) HEK293T cells stably expressing HA-USP37were pretreated with DMSO or 10 μM Ku55933 for 4 h followed by mock treatment or IR (10 Gy). After that, HA-USP37 was immunoprecipitated, left untreated or treated with phosphatase, and immunoblotted with phospho-SQ/TQ (pSQ/TQ) antibody. (C) ATM+/+ or ATM−/−cells were treated with cisplatin (3 μmol/l) for 16 h or left untreated. After that, USP37 was immunoprecipitated, and blots were probed with the indicated antibodies. (D) HEK293T cells stably expressing FLAG-USP37 (WT or S114A) were left untreated or cisplatin (3 μmol/l) for 16 h. Flag-USP37 was immunoprecipitated and blots were probed with the indicated antibodies. (E) HEK293T cells were transfected with the indicated plasmids and untreated or treated with cisplatin (3 μmol/l) for 16 h and MG132 for 4 h to normalize the BLM protein level and thenfollowed by co-IP and western blots. (F) USP37 knockdown HEK293T cells stably expressing the indicated constructs were left untreated or treated with cisplatin (3 μmol/l) for 16 h and MG132 for 4 h. Cells were lysed under denaturing conditionsand then immunoprecipitated with Ni-NTA beads and blotted with indicated antibodies. (G) Purified WT-USP37, the S114A or S114E mutant was incubated with ATM for in vitro kinase assay, and then ATM-phosphorylated USP37 constructs were used for in vitro deubiquitination reaction with ubiquitinated Myc-BLM. The reactions were then blotted with the indicated antibodies. WT: wild type; S114A: phosphorylation-resistant mutant; S114E: the phosphomimicking mutant.