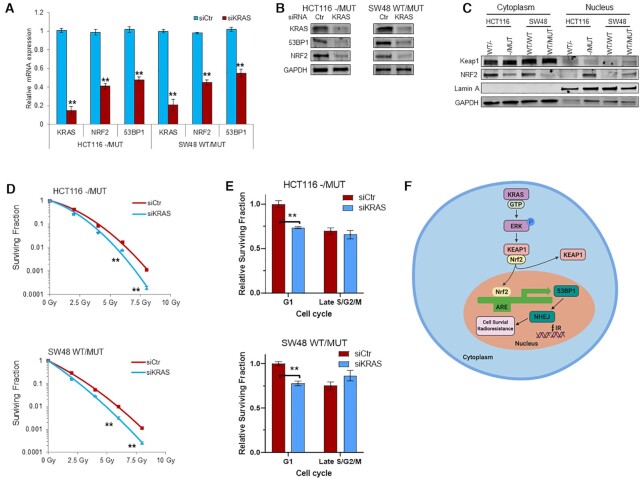
Figure 8.
KRAS mutation promotes NRF2 nuclear expression and KRAS depletion inhibits NRF2-53BP1 expression leading to radiosensitization. (A and B) HCT116 and SW48 G13D isogenic cells were transfected with KRAS siRNA (siKRAS) or control scrambled siRNA (siCtr) for 48 h. The mRNA (A) and protein (B) expression levels of 53BP1, NRF2 and KRAS were determined by qRT-PCR and immunoblotting, respectively. (C) Subcellular fractionation of HCT116 and SW48 isogenic cells reveal higher levels of NRF2 in the nucleus in KRAS MUT cells. (D) Effects of KRAS knockdown by siRNA on radiation sensitivity of HCT116 and SW48 KRAS G13D mutant cells was evaluated by radiation clonogenic assay. (E) Cell cycle enriched clonogenic assay with 2 Gy in HCT116 KRAS mutant (top) and SW48 KRAS mutant (bottom) cells after KRAS genetic silencing. (F) Schematic model of the regulation of DSB repair via KRAS-NRF2-53BP1 signaling axis. Activated (oncogenic) KRAS increases NRF2 translocation into the nucleus, leading to increased binding and transcription of anti-oxidant response element genes, including 53BP1. Increased 53BP1 expression promotes NHEJ repair after ionizing radiation, promoting cell survival and radioresistance; **P < 0.001.