ABSTRACT
Both diabetes mellitus and Charcot–Marie–Tooth disease (CMT) can lead to severe peripheral neuropathy. The differential diagnosis of peripheral neuropathy is difficult due to the similar clinical features. There are still some clues, such as unusual muscle atrophy, unmatched severity of peripheral neurogenic damage with nephropathy or retinopathy, which could alert clinicians to make differential diagnosis. Although diabetes mellitus is rarely concurrent with CMT, it will exacerbate clinical disorders in patients with CMT. To date, there is no specific medicine for CMT treatment. Offloading devices and desirable comprehensive management of diabetes mellitus might be beneficial to avoid plantar ulcer recurrence and anti‐progression of CMT.
Keywords: Charcot–Marie–Tooth disease, diabetic foot, peripheral neuropathy
The differential diagnostic of diabetes peripheral neuropathy and Charcot–Marie–Tooth disease (CMT) is troublesome due to the similar clinical features. Although diabetes mellitus is rarely concurrent with CMT, it will exacerbate clinical disorders of patients with CMT. A desirable comprehensive management of diabetes mellitus might be of benefit to anti‐progression of CMT.

INTRODUCTION
As a severe complication of diabetes mellitus, foot ulcers can lead to a higher amputation rate and increased mortality of patients 1 . The most common risk factor of foot ulcers is known as diabetic peripheral neuropathy (DPN), which could lead to loss of protective sensation and aggravated mechanical foot pressure 2 . The diagnosis of DPN is easy depending on the duration of diabetes, poor glycemic control, clinical features and auxiliary examination (e.g., electromyogram). However, other peripheral nerve diseases, which can be coexistent with diabetes mellitus and aggravate lesions in lower limbs, need to be differentially diagnosed with DPN 3 .
Charcot–Marie–Tooth disease (CMT) is a most common inherited polyneuropathy with a prevalence of 1/2,500 worldwide 4 . The typical clinical features of CMT are characterized by sensory loss, symmetrical distal muscle weakness and diminished deep tendon reflexes. Due to the similar phenotypes with DPN, patients suffering from CMT could merely present with distal symmetrical sensory loss, which makes the differential diagnosis more difficult.
Here, we report a case of diabetes mellitus coexisting with CMT, which presented as a recurrent foot ulcer, but was misdiagnosed as diabetic foot in a Chinese patient.
CASE REPORT
The 38‐year‐old man was diagnosed with type 2 diabetes mellitus based on a fasting glucose level of 16.2 mmol/L in 2013. He did not accept either antidiabetic treatment or blood glucose monitoring. Since 2016, the patient experienced polyuria, numbness of the lower limbs, combined with hypalgesia and loss of temperature sensation. Metformin 500 mg t.i.d. and methyl vitamin B12 (methylcobalamin) 0.5 mg t.i.d. were prescribed, and 6–7 mmol/L for fasting glucose and 8 mmol/L for 2‐h postprandial glucose were obtained. However, he did not feel any improvement of the aforementioned symptoms and suffered from a recurrent foot ulcer on the anterolateral right foot since then. In 2019, the patient suffered from an ulcer on the anterolateral right foot again and was referred to West China Hospital, Chengdu, China.
After admission to hospital, the vital signs were stable, with a blood pressure of 140/86 mmHg. The body examination showed a bilateral pes cavus and hammer‐toe deformity, with plantar desquamation, and callus of bilateral feet (Figure 1a). A plantar ulcer with 1 cm in diameter was observed on the anterolateral right foot (Figure 1b). We also observed a slight atrophy of the thenar and distal lower limbs muscles. The distal muscle strength was graded as 4 for four limbs, accompanied with a normal muscle strength of proximal limbs. The muscle tension was normal, but the reflex of bilateral knee and ankle were not drawn out. The patient was tall and obese, with a body mass index of 33.57 kg/m2. Laboratory tests showed a glycated hemoglobin of 6.2%, with both blood lipids and urinary albumin‐to‐creatinine ratio in the normal range. Fundus photography showed a normal retina. Meanwhile, electromyography showed a peripheral neurogenic damage to four limbs, and severe injuries to motor and sensory fibers, especially in the lower limbs (Table S1). The X‐ray showed osteoporosis for both feet, without bone destruction. Magnetic resonance imaging of lower limbs and feet showed extensive intramuscular fat accumulation, especially in the distal (Figure 1c,d).
Figure 1.
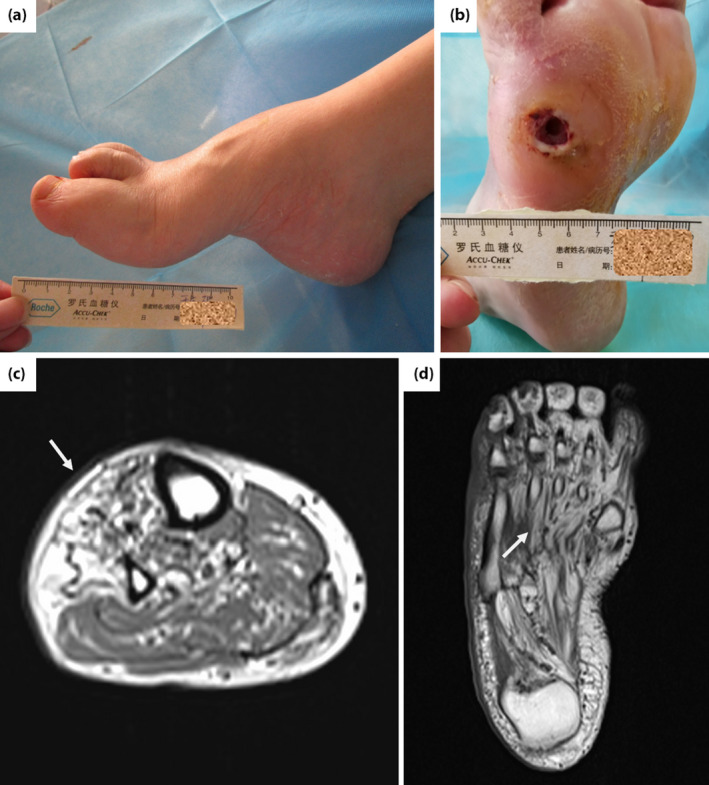
The lesions of feet and magnetic resonance imaging of distal lower limbs and feet. (a) Pes cavus and hammer‐toe; (b) desquamation, callus and plantar ulcer 1 cm in diameter on the anterolateral right foot; extensive intramuscular fat accumulation (white arrow) in (c) the tibialis anterior muscle, peroneus longus and (d) plantar muscle.
Based on the aforementioned information, we took genetic testing for the patient and his parents. A duplication of the peripheral myelin protein 22 (PMP22) gene on chromosome 17p11.2 was detected in both the patient and his father, and a wild‐type gene of PMP22 was detected in his mother. Finally, the patient was diagnosed with CMT type 1A (CMT1A), accompanied with type 2 diabetes mellitus. A removable knee‐high offloading device was used to prevent plantar ulcer relapse. After a year of follow up, the patient’s foot ulcer did not recur to date.
This study was approved by the Ethics Committee on Biomedical Research, West China Hospital of Sichuan University; approval number: 2018年审 (542) 号, Date on which the approval was granted: 12 December 2018.
DISCUSSION
Pes cavus is a common and salient clinical feature for patients with CMT. Nearly half of the patients with pes carvus were finally diagnosed with CMT 5 . However, it is also prevalent in patients with diabetes, which makes it easily misdiagnosed as diabetic foot in patients with diabetes mellitus.
In the present report, the patient was diagnosed with type 1 diabetes mellitus 6 years earlier, and suffered from a recurrent plantar ulcer on the right foot in the past 3 years. Doctors treated him as though he had a common diabetic foot disease. When he was referred to our hospital, we found that the patient showed neither nephropathy nor retinopathy, but a severe peripheral neuropathy, as well as an atrophy of the thenar and distal lower limbs muscles, which were incapably explained by DPN. After gene testing, the patient was finally verified with the CMT1A (PMP22 gene duplication on chromosome 17p11.2) diagnosis.
In all types of CMT, duplication of PMP22 (CMT1A) has been proved to have the highest proportion worldwide 6 . Due to the data from research by Liu et al. 7 , the frequency of CMT1A is nearly 23.3% in mainland China. The phenotype of CMT1A could be a variation even within the same family according to some medical factors, which made it difficult to diagnose. Diabetes mellitus is one of the factors leading to phenotypic difference. Sun et al. 8 reported a Chinese family with coexisting CMT1A and type 2 diabetes mellitus. Seven members were diagnosed with CMT1A by gene testing, four of which with coexisting type 2 diabetes mellitus showed typical symptoms of CMT1A, whereas the other three with no history of diabetes were asymptomatic for CMT1A 8 . This phenomenon was also observed in our report. A positive gene mutation of PMP22 was also detected in the patient’s father, although he was asymptomatic for CMT. The only difference between them was the absence of diabetes mellitus in the father, which indicates the close relationship between diabetes mellitus occurrence and CMT disease progression.
Diabetes mellitus is rarely concurrent with CMT1A, whereas it exacerbates clinical disorders of CMT1A. The mechanism of this connection is still uncertain. Diabetes mellitus does not exacerbate the neuropathy caused by CMT1A directly. Previous animal studies identified that diabetes could result in transmission abnormalities of the neuromuscular junction 9 , which might be a potential mechanism of diabetes mellitus exacerbating CMT1A. It could be possible that diabetes mellitus aggravates pre‐existing demyelinating Schwann cells and axonal structure abnormal interaction, and is associated with regeneration disorder of axons injuries. To date, there is no specific medicine for CMT in clinics 10 . Offloading devices and a desirable comprehensive management of diabetes mellitus might be beneficial to avoid plantar ulcer recurrence and anti‐progression of CMT.
DISCLOSURE
The authors declare no conflicts of interest.
Supporting information
Table S1 | Electromyography results of the patient.
ACKNOWLEDGMENTS
This study was partial supported by National Science and Technology Major Project (Grant No. 2017ZX09304023); West China Nursing Discipline Development Special Fund Project, Sichuan University (Grant No. HXHL20005); Science and Technology Bureau of Sichuan Province (2021JDKP0044); Science and Technology Bureau of Chengdu City (Grant No 2017‐CY02‐00028‐GX); Health Medical Big Data Application and Innovation Project in Sichuan (Grant No. 2018gfgw001); 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. ZYGD18025).
J Diabetes Investig 2021; 11: 2099–2101
REFERENCES
- 1. Bus SA, Armstrong DG, van Deursen RW, et al. IWGDF guidance on footwear and offloading interventions to prevent and heal foot ulcers in patients with diabetes. Diabetes Metab Res Rev 2016; 32: 25–36. [DOI] [PubMed] [Google Scholar]
- 2. Chang HR. Neuropathic diabetic foot ulcers. New Eng J Med 2004; 351: 1694–1695. [DOI] [PubMed] [Google Scholar]
- 3. Pop‐Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017; 40: 136–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barreto LCLS, Oliveira FS, Nunes PS, et al. Epidemiologic study of Charcot‐Marie‐Tooth disease: a systematic review. Neuroepidemiology 2016; 46: 157–165. [DOI] [PubMed] [Google Scholar]
- 5. Nagai MK, Chan G, Guille JT, et al. Prevalence of Charcot‐Marie‐Tooth disease in patients who have bilateral cavovarus feet. J Pediatr Orthop 2006; 26: 438–443. [DOI] [PubMed] [Google Scholar]
- 6. Karakis I, Gregas M, Darras BT, et al. Clinical correlates of Charcot‐Marie‐Tooth disease in patients with pes cavus deformities. Muscle Nerve 2013; 47: 488–492. [DOI] [PubMed] [Google Scholar]
- 7. Liu X, Duan X, Zhang Y, et al. Clinical and genetic diversity of PMP22 mutations in a large cohort of chinese patients with Charcot‐Marie‐Tooth disease. Front Neurol 2020; 11: 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sun A‐P, Tang LU, Liao Q, et al. Coexistent Charcot‐Marie‐Tooth type 1A and type 2 diabetes mellitus neuropathies in a Chinese family. Neural Regen Res 2015; 10: 1696–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Win HHN, Davenport C, Delaney S, et al. Charcot‐Marie‐Tooth disease complicating type 2 diabetes. J Am Podiatr Med Assoc 2011; 101: 349–352. [DOI] [PubMed] [Google Scholar]
- 10. Rossor AM, Shy ME, Reilly MM. Are we prepared for clinical trials in Charcot‐Marie‐Tooth disease? Brain Res 2020; 1729: 146625. 10.1016/j.brainres.2019.146625 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 | Electromyography results of the patient.


