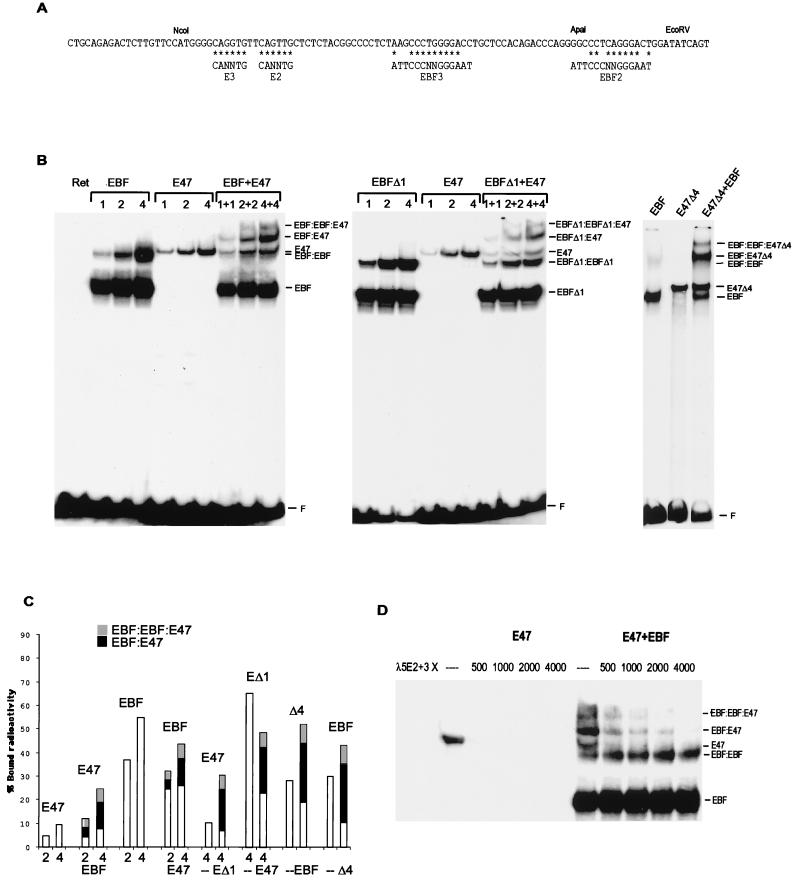
FIG. 6.
EBF and E47 form a stabilized ternary complex on the λ5 promoter. (A) DNA sequence of the 5′ region of the λ5 promoter with indicated EBF binding sites and E-boxes. Asterisks indicate base pair matches to the consensus binding sites. (B) Autoradiograms from EMSA with an end-labeled λ5 5′ NcoI-EcoRV fragment and increasing amounts of in vitro-translated EBF or/and E47 proteins as indicated. The middle panel shows an EMSA with the same probe and increasing amounts of in-vitro-translated EBFΔ1 and/or E47. The amount of protein in each reaction was normalized by the addition of nonprogrammed reticulocyte lysate. The right panel displays an EMSA with the λ5 promoter probe and 7.5 μg of nuclear extracts from transfected HeLa cells in combination with 1.5 μl of in vitro-translated EBF. The gels displayed are representative of two experiments. F, free DNA. (C) A diagram compiling the data obtained by densitometric analysis of the obtained EMSA complexes. The bars represent the total relative amount of radioactivity in complex with protein and are divided into sections according to the presence of the protein in homo- (white) or heteromeric (black and grey) complexes as indicated. (D) Autoradiogram of an EMSA where the preformed complexes between E47 and/or EBF on the λ5 promoter have been distorted by the addition of increasing amounts of unlabeled oligonucleotides spanning the E2 and E3 boxes in the λ5 promoter as indicated. The gel displayed is representative of three experiments. The probe is not shown, since the gel has been cut to save space.