Figure 3.
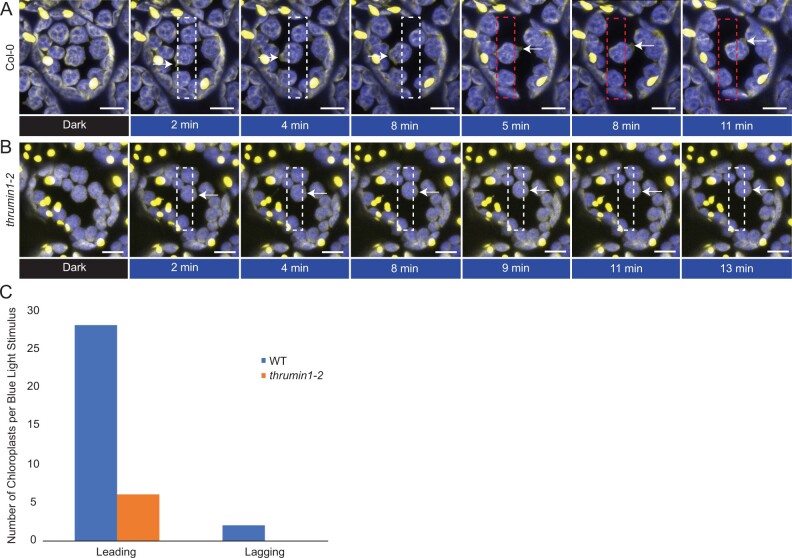
Chloroplast membranes display directional amoeboid-like movements in response to blue light. A, Col-0 WT plants expressing the stroma-localized marker tpFNR:YFP were imaged in a dark state (YFP excitation, 514 nm) and a blue-light-stimulated region of interest (white rectangle, 470 nm). As per normal chloroplast movement kinetics, within a minute of blue-light stimulation, the chloroplasts began to leave the region of interest with a membrane protrusion in the direction of movement (white arrows). When the region of blue-light stimulation is altered to force a new direction of chloroplast movement (red rectangle), a new membrane protrusion in the direction of movement is formed (white arrows at 5+ min). Images are representative frames from Supplemental Movie S3. B, thrumin1-2 mutant plants expressing the tpFNR:YFP marker did not exhibit robust membrane protrusions (white arrows) in response to a blue-light stimulus (white rectangle). The chloroplasts moved in slow but sporadic patterns with no discernable membrane activity after 13 min of a blue-light stimulus. Images are representative frames from Supplemental Movie S5. A and B, Chlorophyll autofluorescence is false-colored blue and the YFP channel is false-colored yellow. Representative time-lapse images are shown for the dark treatment (514 nm for YFP excitation) and blue-light stimulation intervals (470 nm and 514 nm). The scale bars indicate a 5-µm distance. C, THRUMIN1 association is with the development of chloroplast membrane protrusions on the leading edge of chloroplasts in response to high blue-light microbeam irradiation. Chloroplast membrane protrusion events were counted along the leading and lagging edges in WT and thrumin1-2 mutant backgrounds. The histograms show the total leading/lagging edge protrusion events for 31 chloroplasts from 8 different cells for WT and 30 chloroplasts from 9 different cells for the thrumin1-2 mutant.