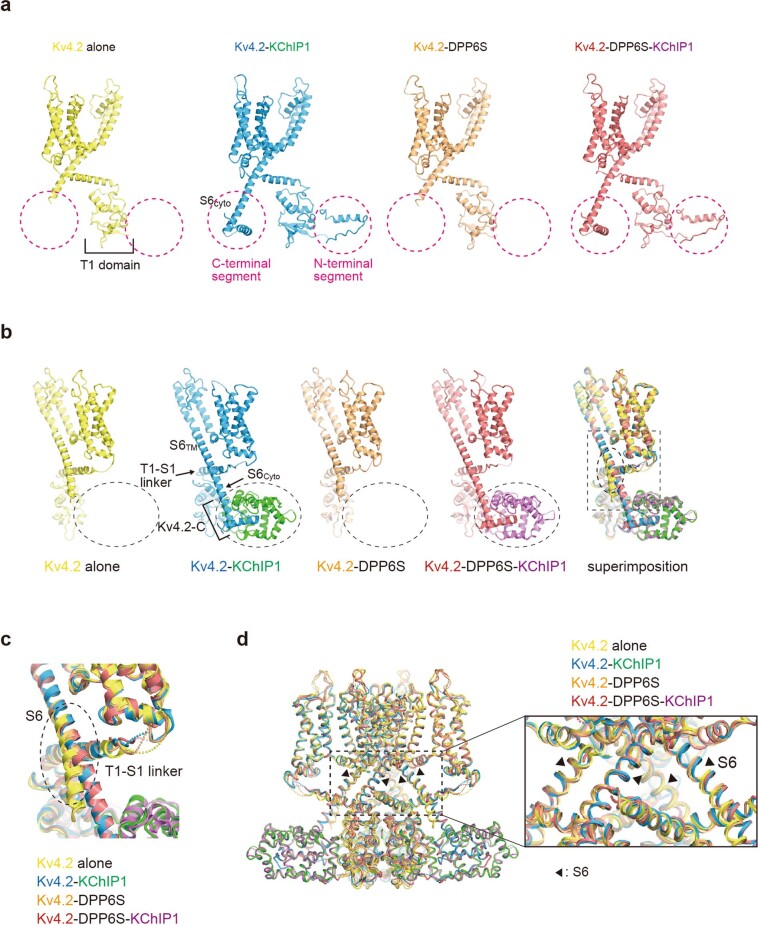
Extended Data Fig. 11. Structural comparison of N- and C-terminal conformations in the presence and absence of KChIP1.
a. Structural comparison of the Kv4.2 protomers from Kv4.2 alone, Kv4.2–KChIP1, Kv4.2–DPP6S, and Kv4.2–DPP6S–KChIP1, showing that both the N- and C-terminal regions are disordered in the absence of KChIP1 as observed in the structure of Kv4.2–DPP6S and Kv4.2 alone. Both terminal regions are resolved in the structure of Kv4.2–DPP6S–KChIP1 and Kv4.2–KChIP1. b. Comparison of the Kv4.2 S6 conformations. The intracellular S6 helices of Kv4.2–DPP6S and Kv4.2 alone bend at the interface on the T1-S1 linker (dashed ellipse in the superimposed image) and is subsequently disordered. By contrast, the S6 helices of Kv4.2–DPP6S–KChIP1 and Kv4.2–KChIP1 complexes extend straight toward KChIP1. c. Close-up view of the superimposed image in the dashed box in (b). The intracellular S6 of Kv4.2 bend and extend away from the T1-S1 linker in the Kv4.2–DPP6S complex and Kv4.2 alone. However, it keeps a close distance to T1-S1 linker without bending in the Kv4.2–DPP6S–KChIP1 and Kv4.2–KChIP1 complexes. d. Superimposition of the four Kv4.2 structures reveals that the S6 helices adopt an open conformation in all structures.