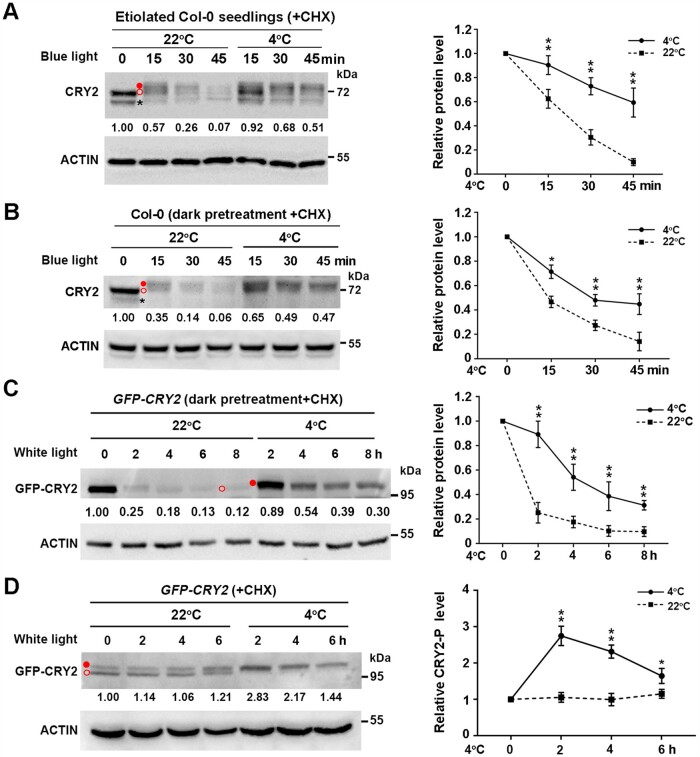
Figure 3.
Cold promotes the stability of phosphorylated CRY2 induced by blue light. A, Effects of cold exposure on CRY2 protein levels in 10-day-old etiolated wild-type Col-0 seedlings transferred to blue light (20 µmol m−2 s−1) for the indicated time. Seedlings were treated with 300 µM CHX for 0.5 h, followed by transfer to 4°C or back to 22°C in the dark or in blue light in the presence of CHX. B and C, Effects of cold exposure on CRY2 protein levels in Col-0 seedlings and GFP-CRY2 transgenic lines. A 13-day-old LD-grown Col-0 and GFP-CRY2 transgenic seedlings were pretreated in the dark at 22°C for 24 h, treated with 300 µM CHX for 0.5 h, followed by transfer to 4°C or back to 22°C for the indicated times in blue light (B) or white light (C) (20 µmol m−2 s−1). D, Effects of cold exposure on GFP-CRY2-P protein levels in GFP-CRY2 transgenic seedlings in constant white light (20 µmol m−2 s−1). A 13-day-old LD-grown GFP-CRY2 seedlings were treated with 300 µM CHX for 0.5 h at ZT2 (Zeitgeber 2; 2 h after dawn), followed by treatment at 22°C or 4°C for the indicated times in constant white light (20 µmol m−2 s−1). In (A–D), total proteins were extracted and subjected to immunoblot analysis with anti-CRY2 (A and B) or anti-GFP (C and D) antibodies to detect CRY2 protein. Actin served as a loading control. Immunoblot results were quantified using ImageJ software. Relative protein levels are shown to the right of the blots, with relative protein levels at 0 h set to 1.00. Quantitative data are means of three independent experiments ±sd. Asterisks indicate significant differences compared with 22°C under the same light conditions (*P < 0.05, **P < 0.01, Student’s t test). Open red circle, nonphosphorylated CRY2; filled red circle, phosphorylated CRY2; asterisk, nonspecific band.