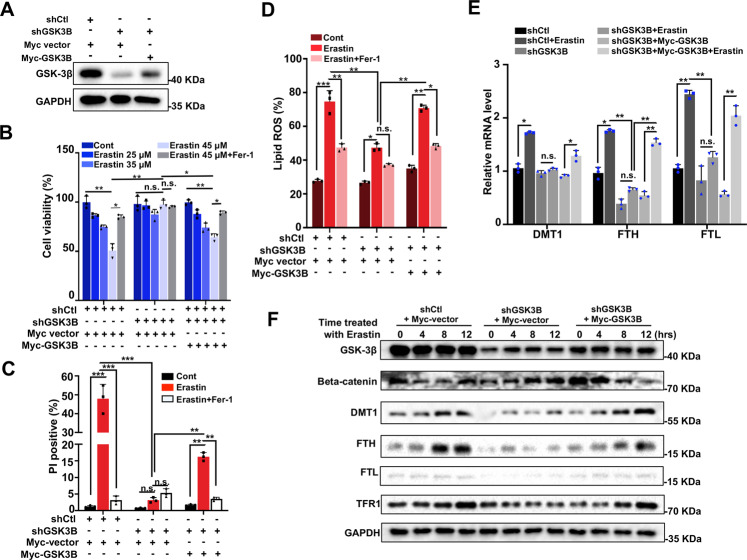
Fig. 5. Re-expression of GSK-3β restores GSK-3β depletion-resisted ferroptosis.
A Indicated shCtl or shGSK-3β HeLa cells were transfected with either control plasmid (myc-vector) or myc-GSK-3β plasmid. Re-expression of GSK-3β was confirmed by immunoblotting. B shCtl or shGSK-3β HeLa cells transfected with myc-vector or myc-GSK-3β were treated with erastin (35 μM) with or without Fer-1 (20 μM) for 24 h, cell viability was assayed using a CCK8 kit. C shCtl or shGSK-3β HeLa cells transfected with myc-vector or myc-GSK-3β were treated with erastin (35 μM) with or without Fer-1 (20 μM) for 24 h, and cell death was measured by propidium iodide (PI) staining using fluorescence microscopy. The percentage of PI positive (red) cells were quantitated. Data shown represent mean ± SD from three independent experiments. D shCtl or shGSK-3β HeLa cells transfected with myc-vector or myc-GSK-3β were treated with erastin (35 μM) with or without Fer-1 (20 μM) for 24 h, and lipid ROS production was detected by flow cytometry using C11-BODIPY. The percentage of BODIPY-positive cells in C were quantitated. Data shown represent mean ± SD from three independent experiments. E shCtl or shGSK-3β HeLa cells transfected with myc-vector or myc-GSK-3β were treated with 35 μM erastin for 24 h, and the mRNA expression of DMT1, FTH1, and FTL in indicated HeLa cells was assayed by RT-qPCR. The relative gene expression is normalized to GAPDH. Data shown represent mean ± SD from three independent experiments. F Indicated shCtl, shGSK-3β, and shGSK-3β transfected with myc-GSK-3β HeLa cells were treated with 35 μM erastin for different times (0, 4, 8, 12 h) as indicated, and subjected to Western blotting for GSK-3β, pGSK-3β (Ser9), beta-catenin, DMT1, FTH, FTL, and TFR1. Statistical analysis was made using Student’s t-test; *p < 0.05, **p < 0.01, ***p < 0.001.