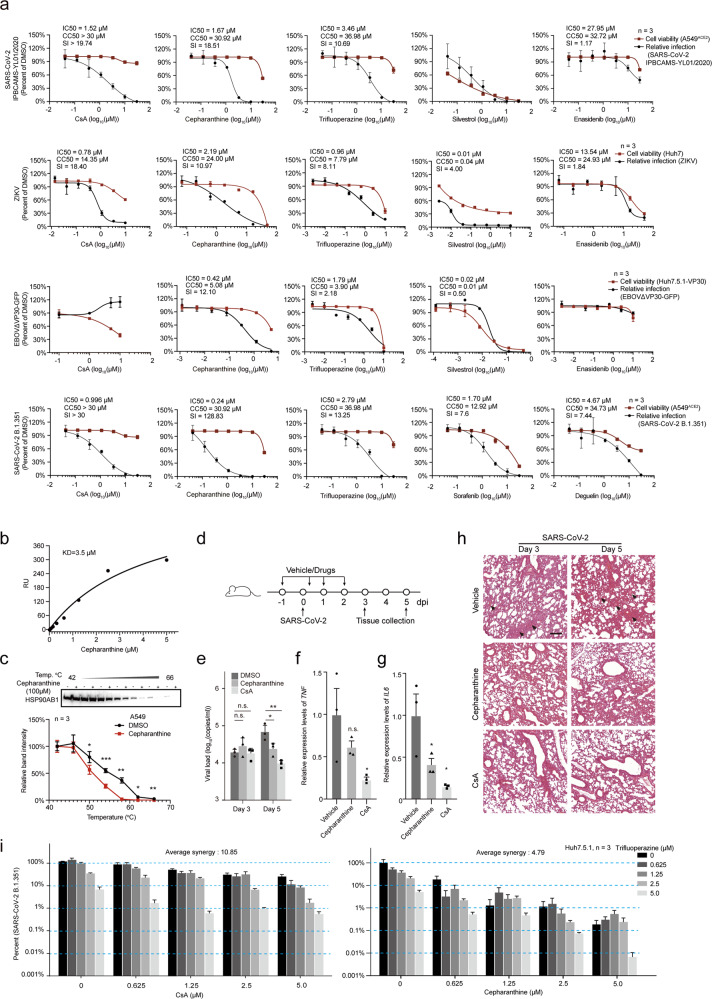
Fig. 5. Validation of antiviral activities of FDA-approved and clinical-trial drugs targeting vRNA-interacting proteins.
a The antiviral activities of the indicated drugs against infection with SARS-CoV-2 (IPBCAMS-YL01/2020) (first row), ZIKV (MR766) (second row), EBOVΔVP30-GFP (third row), and the B.1.351 SARS-CoV-2 variant (fourth row), at different drug concentrations. Red line, cell viability; black line, infection ratio relative to the vehicle control (DMSO) group. Cell lines used for infection assay were indicated on the right. Data are means ± SD. n = 3 biologically independent samples. IC50, CC50 and SI values are indicated. b Surface Plasmon Resonance (SPR) to validate the binding of Cepharanthine to HSP90AB1. About 8–10 μg of purified HSP90AB1 proteins were coupled to a CM5 sensor chip (GE healthcare) with an immobilization level of ~15000 RU. Different concentrations of Cepharanthine were injected on the chip, and the affinity constant (KD) was tested using SPR. RU, relative response unit. c Target engagement assay of Cepharanthine. CETSA showed that Cepharanthine targeted HSP90AB1. Top, western blot, HSP90AB1 protein was detected using a specific antibody after the A549 cells were treated with Cepharanthine (100 μM) or DMSO (1%, as control) and heated. Bottom, quantification of the western blot. Data are means ± SD, n = 3 biologically independent samples. ***P < 0.001, **P < 0.01, *P < 0.05. Two-tailed student’s t-test. d Schematic for virus infection and drug administration for mice. Drug or vehicle (2% DMSO, 30% PEG-300, and 5% Tween-80) was administered intranasally to hACE2 transgenic mice (Cepharanthine or CsA, 10 mg/kg) one day before viral challenge. The mice were subsequently subjected to intranasal challenge with SARS-CoV-2 (with 105 TCID50, IPBCAMS-YL01/2020). Lung tissues were sampled at the indicated time points. n = 3 mice were euthanized at 3 dpi and 5 dpi respectively, for each drug and vehicle control group. e Antiviral effects of Cepharanthine and CsA against SARS-CoV-2 in vivo. As in d, 6 hACE2 mice were infected with SARS-CoV-2 and treated with Cepharanthine, CsA or the DMSO vehicle solution, and were sacrificed at 3 or 5 dpi. Viral loads in the lungs of these mice were quantified by qPCR. Data are shown as means ± SEM. *P < 0.05, **P < 0.01. Two-tailed student’s t-test. f, g The expression of pro-inflammatory cytokines in lungs of SARS-CoV-2-infected mice. As in d, the hACE2 transgenic mice were infected with SARS-CoV-2 and treated with Cepharanthine or CsA. The expression levels of TNF (f) and IL6 (g) in mouse lung relative to those in the vehicle treatment group were quantified by qPCR at 5 dpi. For f, g, n = 3. Data are shown as means ± SEM, *P < 0.05, Two-tailed student’s t-test. h H&E staining of lung tissues of mice infected with SARS-CoV-2. As in d, upon SARS-CoV-2 infection, mice were intranasally given Cepharanthine or CsA (10 mg/kg daily). Lung tissues were collected at 3 dpi and 5 dpi for H&E staining assay. Fluid exudates and inflammations are indicated by black arrow. Scale bar, 200 μm. i Antiviral effects of co-treatment with CsA and Trifluoperazine (left), or Cepharanthine and Trifluoperazine (right). CsA or Cepharanthine was combined with Trifluoperazine as the indicated concentrations. Huh7.5.1 cells were treated with the combined drugs and infected with the B.1.351 strain of SARS-CoV-2 (MOI 0.05). Cells treated with vehicle without drugs (DMSO) were used as control. Viral loads in culture supernatants were quantified at 48 h post infection by qPCR (represented as percentage relative to the DMSO-treated samples). The highest single agent model72,73 was used to assess the pharmacological interactions (average synergy) between Trifluoperazine and CsA (left), and between Trifluoperazine and Cepharanthine (right). Data are means ± SEM. n = 3 biologically independent samples.