FIGURE 3.
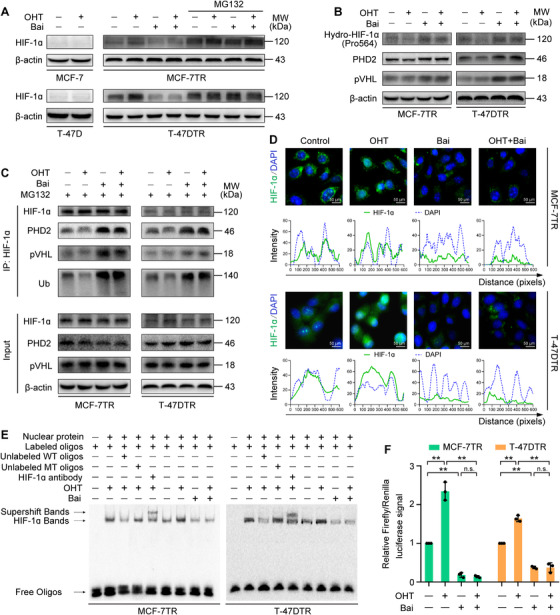
Baicalein reduces the expression and transcriptional activity of HIF‐1α in TAM‐resistant cells. Parental and TAM‐resistant cells were treated with OHT (1 μM), baicalein (25 μM), or OHT (1 μM) combined with baicalein (25 μM) for 48 h. TAM‐resistant cells were pretreated with 10 μM MG132 for 12 h and then treated as mentioned above. (A) HIF‐1α expression was detected via western blotting. (B) Expression levels of prolyl‐hydroxylated‐HIF‐1α (Pro564), PHD2 and pVHL were detected via western blotting. (C) Cell lysates were immunoprecipitated with HIF‐1α antibody. Immunoprecipitated materials and total cell extracts were analysed via western blotting with specific antibodies for ubiquitin (Ub), PHD2 and pVHL. Immunoprecipitated HIF‐1α was used as the loading control. (D) Subcellular localization of HIF‐1α was confirmed by immunofluorescence staining. HIF‐1α is represented by green fluorescence and cell nuclei were stained with DAPI (blue fluorescence; magnification, 400 × ; scale bars, 50 μm). Fluorescence intensity distribution curves show superposition of green fluorescence (HIF‐1α) and blue fluorescence (DAPI), confirming nuclear localization of HIF‐1α. (E) Baicalein inhibits the binding of HIF‐1α to a biotin‐labelled HRE consensus oligonucleotide in the presence or absence of OHT treatment. A specific anti‐HIF‐1α antibody was used to confirm HIF‐1α–HRE formation via a supershifted complex. Unlabelled wild‐type (WT) and mutant‐type (MT) probes were used as competitor oligonucleotides . (F) HRE‐luciferase reporter activity was measured using a dual‐luciferase assay. HRE‐luciferase reporter plasmids (pGL3‐HRE) and pRL Renilla luciferase control plasmids (ratio 10:1) were transfected into cells. After transfection, the cells were treated as mentioned above, and the dual‐luciferase assay was then performed using lysed cells (n = 3). Results are represented as mean ± SD. *p < 0.05, **p < 0.01, control vs. OHT; control vs. baicalein (Bai); OHT vs. OHT plus baicalein; baicalein vs. OHT plus baicalein
